VI. O-Thiocarbonyl Compounds
- Page ID
- 24013
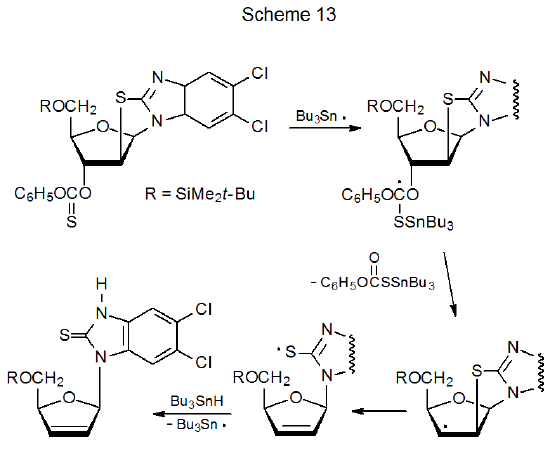
Compounds with carbon–sulfur single bonds are substantially less reactive with tin- and silicon-centered radicals than are compounds with C–S double bonds. Among carbohydrates these double bonds are almost always part of O-thiocarbonyl groups. (The reactions of O-thiocarbonyl carbohydrate derivatives are discussed in Chapter 12.) The reaction shown in eq 6 illustrates the greater reactivity of a C–S double bond when compared to a C–S single bond because only the O-thiocarbonyl group in the 1‑thioglycoside 12 reacts even though an ethylthio group is present in the molecule.39 Greater reactivity of a carbon–sulfur double bond also can be seen in the reaction shown in Scheme 13, where Bu3Sn· reacts only with the O‑thiocarbonyl group.26 A quantitative measure of the reactivity of C–S single and double bonds comes from comparing absolute rate constants for their reactions; thus, rate constants for reaction of (Me3Si)3Si· with C10H21SC6H5 and C6H11OC(=S)SMe are less than 5 x 106 and 1.1 x 109 M-1s-1, respectively, at 21 oC.40 The reactions in Schemes 12 and 13 also illustrate the ease of fragmentation of a carbon–sulfur single bond when a radical is centered on an adjacent carbon atom.
.png?revision=1&size=bestfit&width=345&height=127)