III. Tellurides
- Page ID
- 24019
The organotellurium compounds that are used as radical precursors in carbohydrate chemistry usually are synthesized by a nucleophilic displacement reaction such as that pictured in eq 14.79
.png?revision=1&size=bestfit&width=365&height=103)
The majority of compounds prepared in this way are anomeric tellurides. Furanosyl tellurides are relatively unstable and tend to decompose within a few days,80–82 but although their pyranosyl counterparts can exist unchanged in the solid state for months,79 heating or exposing pyranosyl tellurides to UV light causes epimerization (eq 15).83
.png?revision=1&size=bestfit&width=430&height=101)
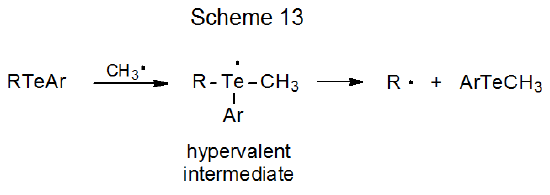
Two procedures, both of which involve photolysis, cause radical reaction of carbohydrate tellurides. The first of these is photochemical homolysis of a carbon–tellurium bond, a reaction that generates the more stable of the two possible, carbon-centered radicals (Scheme 11).
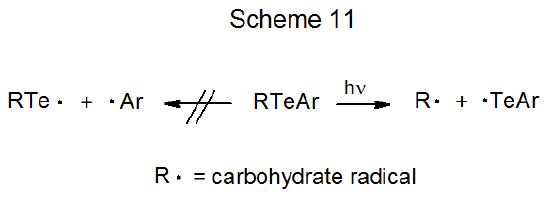
An example of reaction brought about in this way is found eq 16.83 The second procedure for radical formation from a carbohydrate telluride calls for photochemical decomposition of N‑acetoxy-2-thiopyridone to produce a methyl radical that then reacts with the telluride (Scheme 12).
.png?revision=1&size=bestfit&width=375&height=101)
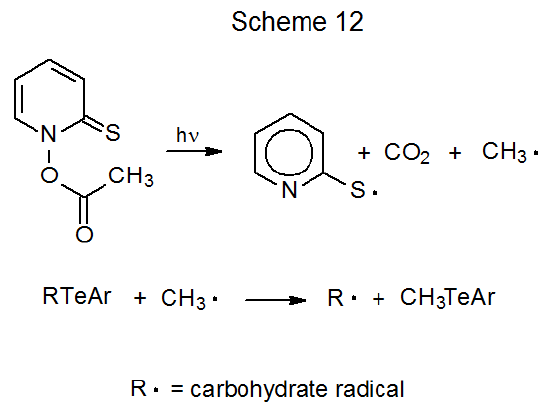
Equation 17 describes a cyclization reaction initiated in this way.84 Reactions of carbohydrate radicals formed from tellurides include cyclization,84,85 addition,86–89 reduction,83 and group migration.83 It is reasonable to assume that reaction of a carbohydrate telluride with a methyl radical involves, as molecular orbital calculations indicate, formation of an intermediate with a hypervalent tellurium atom (Scheme 13).90
.png?revision=1&size=bestfit&width=430&height=226)