III. Thiol-Catalyzed Reactions of Acetals: Polarity-Reversal Catalysis
- Page ID
- 24023
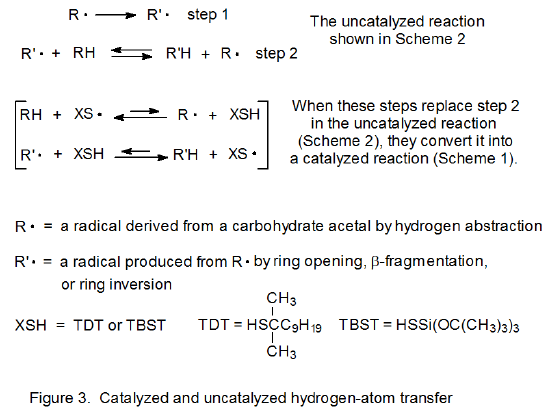
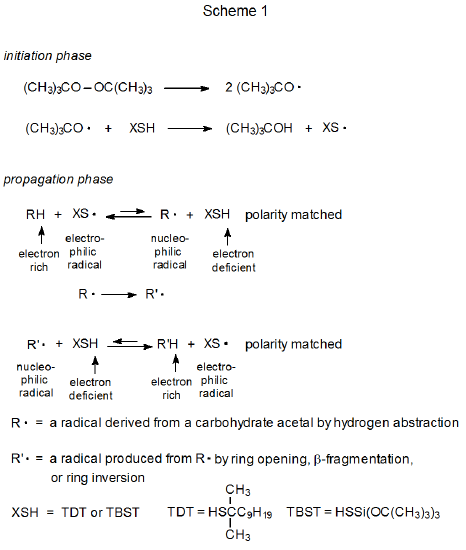
Thiols act as catalysts for hydrogen-atom abstraction from acetals.1–10 The initiation phase in these reactions generates a thiyl radical that then abstracts a hydrogen atom from the acetal in the first propagation step (Scheme 1). This first step is reversible and a pseudo equilibrium is established that favors the reactants (RH and XS·). (The position of this equilibrium is based on the enthalpy calculated from the bond-dissociation energies given in eq 1.11) The overall process is driven by the second propagation step, a reaction that irreversibly converts one carbon-centered radical (R·) into another (R’·). The final step is rapid hydrogen-atom abstraction from the thiol by the newly formed, carbon-centered radical R’.
.png?revision=1&size=bestfit&width=390&height=79)
Hydrogen-atom abstraction from a molecule of substrate should have a lower transition-state energy when the abstracting radical is sulfur-centered (Scheme 1) rather than carbon-centered (Scheme 2). In reactions of this type the transition state can be described as a hybrid of valence-bond structures 1-4 (Figure 1).10 If the abstracting radical is carbon-centered, structures 1 and 2 are the major contributors to the hybrid; the charge-separated structures 3 and 4 are of little consequence. If, however, abstraction is done by a thiyl radical, not only are structures 1 and 2 important, but contribution from the charge-separated structure 3 also is significant.10 (In the case where a thiyl radical abstracts a hydrogen atom from an acetal, the valence-bond structures 1-4 can be represented in the more descriptive manner shown in Figure 2.) Significant contribution from 3 means that the energy required to reach the transition state for abstraction of a hydrogen atom is less than that needed when a carbon-centered radical abstracts the same hydrogen atom. Faster hydrogen-atom abstraction in propagation step 1 (Scheme 1) means that as R· is converted into R’· in step 2, the R· needed to continue the propagation sequence will be replenished more rapidly.
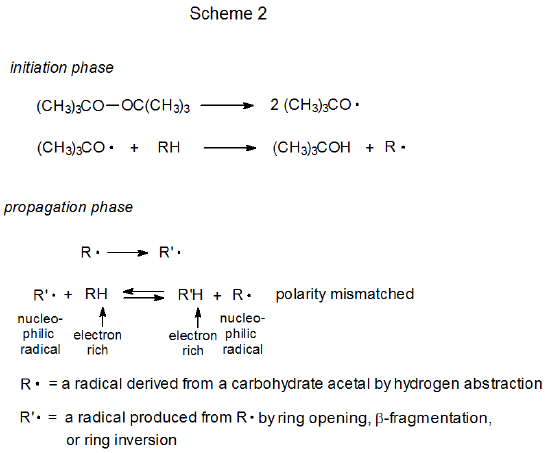
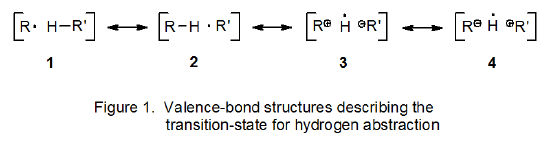
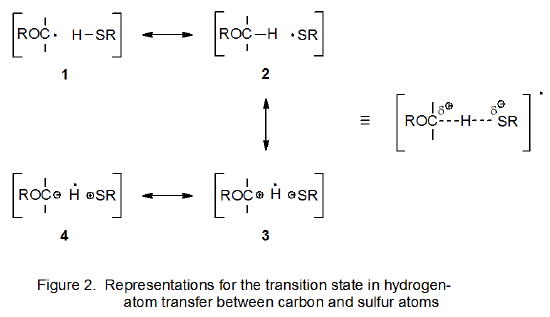
A structure such as 3 is a transition-state-stabilizing contributor in any hydrogen-abstraction reaction where a change in radical philicity takes place. Such a change occurs in propagation steps 1 and 3 in the thiol-catalyzed mechanism pictured in Scheme 1, but it does not take place at all in the uncatalyzed mechanism shown in Scheme 2. When a change in radical philicity occurs during a reaction, either due to abstraction of an electron-rich hydrogen atom by an electrophilic radical or abstraction of an electron-deficient hydrogen atom by a nucleophilic radical (propagation steps 1 and 3, respectively, in Scheme 1), the reaction is described as polarity-matched.4,10 If one radical must be converted into another by hydrogen-atom abstraction without benefit from a change in radical philicity (step 2 in Scheme 2), the reaction is described as being polarity-mismatched. The transition state for a polarity-matched reaction will be stabilized by contribution from the charge-separated, valence-bond structure 3 (Figures 1 and 2), but a polarity-mismatched reaction will not experience similar, transition-state stabilization. A polarity-matched reaction, therefore, will have transition-state stabilization that is denied to a polarity-mismatched reaction.
Although the combination of steps 1 and 3 in Scheme 1 achieves the same result as step 2 in Scheme 2 (see Figure 3), the polarity-matched steps in Scheme 1 can be fast enough that in combination they are more rapid, sometimes much more rapid, than the single, polarity-mismatched step in Scheme 2. When this occurs, the added thiol is said to catalyze the entire reaction by polarity-reversal catalysis.10 The next three sections describe reactions that either are made possible by or have improved yields due to polarity-reversal catalysis.