IV. Ring Opening of Specially Designed Acetals
- Page ID
- 24024
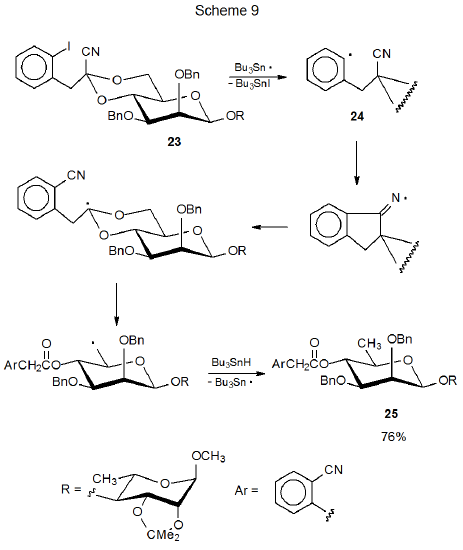
Search for compounds with more versatile reactivity than that provided by a 4,6-O-benzylidene group has stimulated development of some specially designed structures.14–18 The acetal 23, which fits into this “specially designed” category, reacts with Bu3Sn· to form the aryl radical 24. The iodine-atom abstraction that generates 24 is the first step in a sequence of radical reactions that culminates in producing the protected glycoside 25 (Scheme 9).14–16 An example of the synthetic usefulness of this reaction is found in the conversion of a tetrasaccharide containing four such protecting groups into one in which each group is transformed into an O-benzoyl group.15 The glycoside 26 is another cyclic benzylidene acetal with an aromatic iodo substituent that undergoes a sequential radical reaction that leads to the corresponding deoxy benzoate 27 (eq 10).17 The reactions pictured in Scheme 9 and eq 10 are two more examples (in addition to those shown in equations 2 and 3) where trans-fused rings open to produce primary rather than a secondary radicals. Ring opening of the 4,6-O-benzylidene acetal 28 to give a secondary radical (eq 11) further supports the proposal made for the acetal 11 (eq 4) that for a more flexible, cis-fused ring system the direction of ring opening is controlled by radical stability rather than ring strain at the transition state.
.png?revision=1&size=bestfit&width=435&height=130)
.png?revision=1&size=bestfit&width=410&height=214)

