VI. Radical Cyclization: The Role of Ethers and Acetals
- Page ID
- 24026
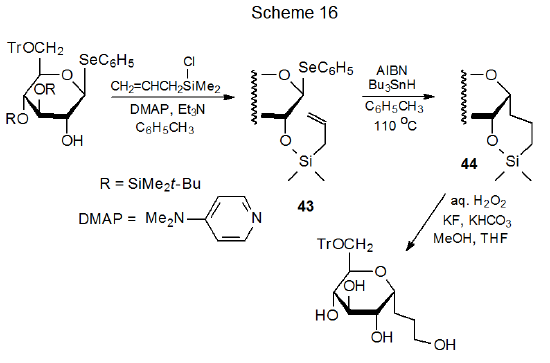
Radical cyclization depends upon having a radical center and multiple bond held in close enough proximity for internal addition to take place. In carbohydrates an ether linkage often is the means for connecting these two reactive centers. In the reaction shown in Scheme 16, for example, a carbohydrate (43) with a radical precursor at C-1 is connected by a silyl ether linkage at C-2 to a substituent containing a double bond.32 Radical cyclization to give the silyl ether 44 creates a new carbon–carbon bond. Nonradical ring opening of 44 produces a silicon-free carbohydrate with an extended, carbon-atom chain.
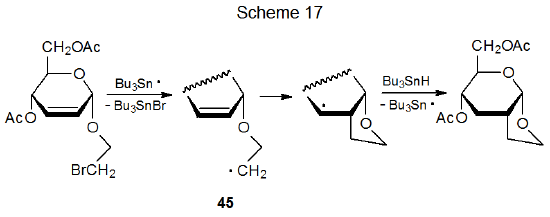
Acetals and nonsilyl ethers also act as tethers that connect reactive centers during radical cyclization. In the reaction shown in Scheme 17, for example, the acetal linkage holds the double bond and the radical center in 45 close enough for ring formation to occur.33 In a similar manner an ether linkage connects the reactive centers during the cyclization reaction shown in eq 13.34 Unlike silyl ethers, the rings formed when acetals and nonsilyl ethers act as tethers usually are not destined for immediate ring opening. (Section IV.C of Chapter 19 contains additional examples of ethers and acetals serving as tethers in radical cyclization reactions.)
.png?revision=1&size=bestfit&width=405&height=164)

