II. Radicals That Abstract Hydrogen Atoms from Unprotected Carbohydrates
- Page ID
- 24034
A. Hydroxyl Radicals
1. Ionizing Radiation and Ultraviolet Light
γ-Radiolysis of water produces hydroxyl radicals along with the compounds, ions, and other radicals shown in eq 1.1–4 The yield of hydroxyl radicals in this reaction can be increased by adding N2O to the reaction mixture because hydrated electrons react with N2O to form hydroxyl radicals (eq 2).2–4 In N2O-containing solutions 85% of the radicals are HO· and 15% are H·.4 Both HO· and H· abstract hydrogen atoms from carbon–hydrogen bonds.4 Hydroxyl radicals (and hydrogen atoms) also can be produced by photolysis of water with ultraviolet light of wavelength less than 185 nm (eq 3).1
.png?revision=1&size=bestfit&width=415&height=32)
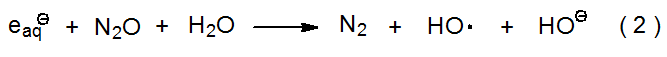.png?revision=1&size=bestfit&width=335&height=29)
.png?revision=1&size=bestfit&width=190&height=31)
2. Reaction of H2O2 with Fe2+ and Ti3+
Reaction of H2O2 with Fe2+ (eq 4) or Ti3+ (eq 5) produces hydroxyl radicals.5–8 (These reagent combinations are sometimes described as radiomimetic, that is, imitating radiation.) Each hydroxyl radical produced is capable of abstracting a hydrogen atom from a carbon–hydrogen bond present in a molecule of substrate (eq 6). The Ti4+ generated by the reaction shown in eq 5 does not react further with the carbon-centered radical produced, but the Fe3+ formed in the reaction shown in eq 4 does;8 Fe3+ oxidizes an α-hydroxy radical to a carbonyl group while itself being reduced to Fe2+. Regeneration of Fe2+ from Fe3+ by the reaction shown in eq 7 means that when this reaction occurs, only a catalytic amount of Fe2+ may be necessary for complete decomposition of H2O2 (eq 4). (The combination of H2O2 and Fe2+ is known as Fenton’s reagent,9 and that of H2O2 and Te3+ as a Fenton-type reagent.8)
.png?revision=1&size=bestfit&width=295&height=29)
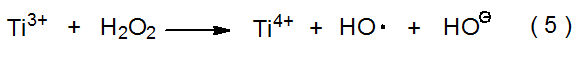.png?revision=1&size=bestfit&width=290&height=29)
.png?revision=1&size=bestfit&width=330&height=54)
.png?revision=1&size=bestfit&width=375&height=45)
B. Sulfate Radical Anions
The sulfate radical anion (SO4·-) forms when the peroxydisulfate dianion (S2O82-) reacts with Ti3+ (eq 8).10 A low concentration of Cu2+ present in the reaction mixture enhances the rate of generation of SO4·- via the reactions shown in equations 9 and 10. The sulfate radical anion also forms from direct photolysis of S2O82- (eq 11).10
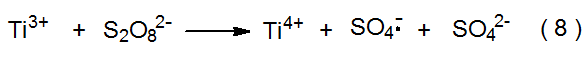.png?revision=1&size=bestfit&width=295&height=29)
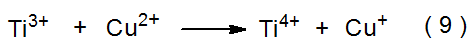.png?revision=1&size=bestfit&width=235&height=26)
.png?revision=1&size=bestfit&width=300&height=32)
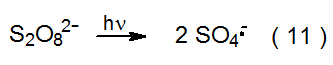.png?revision=1&size=bestfit&width=170&height=33)

