VI. Oxidative Degradation of Carbohydrates
- Page ID
- 24038
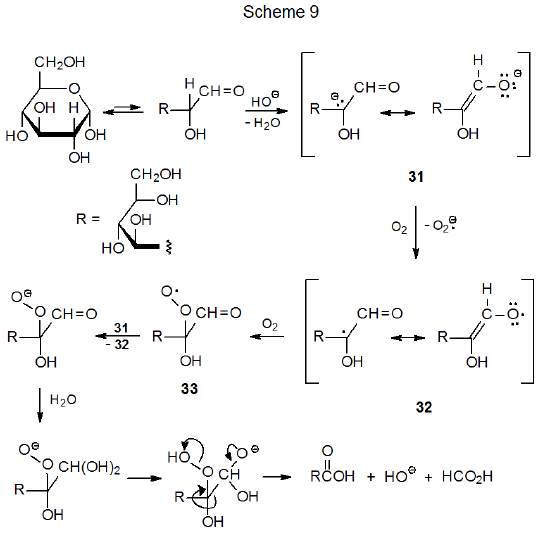
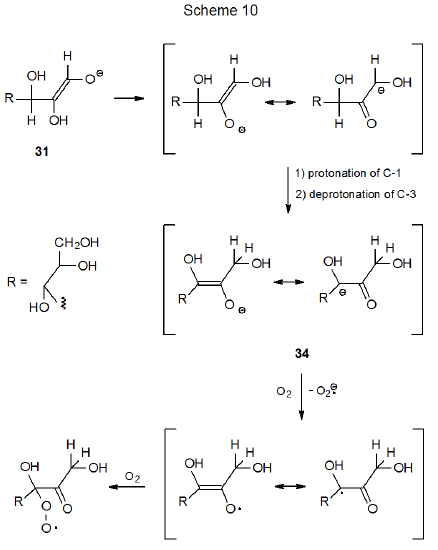
The oxidative degradation of carbohydrates in the presence of base is another reaction that involves radical intermediates. Such reaction of D‑glucose begins with ring opening and deprotonation to give the enediolate anion 31 (Scheme 9).31,32 Oxidation of this anion with O2 produces the resonance stabilized radical 32, which then is converted to the peroxy radical 33 by addition of O2. Subsequent reduction of 33 gives an anion that ultimately fragments the C1–C2 bond to give a five-carbon aldonic acid (Scheme 9). Fragmentation of other carbon–carbon bonds also takes place because base-catalyzed isomerization of the 1,2-enediolate anion 31 produces the 2,3-enediolate anion 34 (Scheme 10). Reaction of 34 with O2 and fragmentation analogous to that shown in Scheme 9 cleaves the D-glucose structure into two-carbon-atom and four-carbon-atom carboxylic acids. Continued isomerization of this type (31 to 34) produces other enediolates that undergo similar fragmentation reactions.32