II. Replacement of an Acyloxy Group with a Hydrogen Atom
- Page ID
- 24042
A. α-Acyloxy Ketones
α‑Acyloxy ketones react with tri-n-butyltin hydride by replacing the acyloxy group with a hydrogen atom (eq 1).1 The importance of the carbonyl group to this replacement process is evident in the two reactions shown in eq 2. In the first of these a benzoate (1) containing a keto group forms a deoxy sugar in good yield, but in the second a benzoate (2) lacking such a group is unreactive.1 Even though reactions of α-acyloxy ketones lead to formation of deoxy sugars, the usefulness of such reactions is limited by the relatively small number of carbohydrates that either have the necessary substituents or easily can be converted into compounds that do.1,2
.png?revision=1&size=bestfit&width=410&height=146)
.png?revision=1&size=bestfit&width=425&height=191)
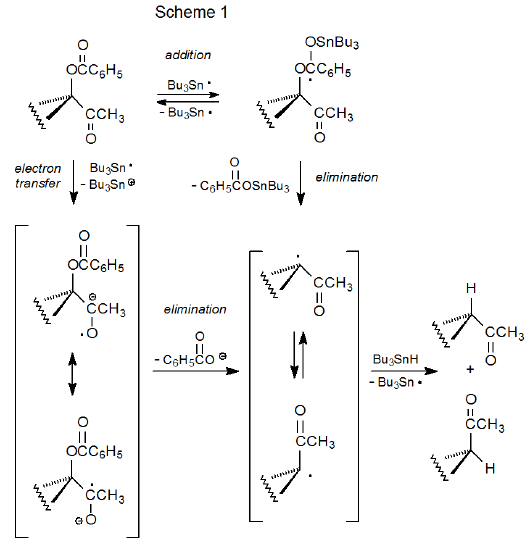
A proposed mechanism for group replacement in α-acyloxy ketones is pictured in Scheme 1. Both addition/elimination and electron-transfer/elimination sequences are presented as possibilities for acyloxy group loss. The addition-elimination possibility was proposed at the time of the discovery of this reaction,1 but the electron-transfer option was recognized as a viable alternative later when loss of the benzoyloxy group from α-(benzoyloxy)acetophenone was shown to involve electron transfer from Bu3Sn· to this α-acyloxy ketone.3 There is no decisive evidence favoring either mechanism.
B. Methyl Oxalyl Esters
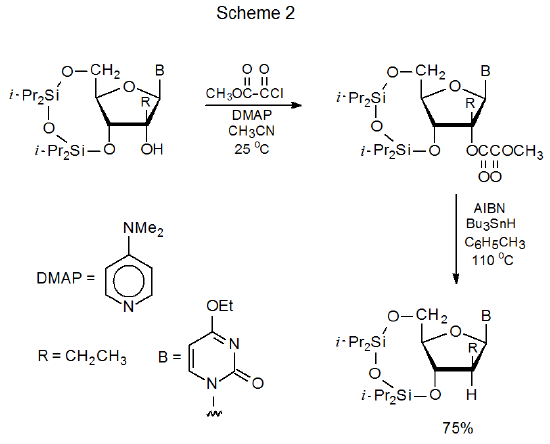
Methyl oxalyl esters can be prepared easily by esterification of partially protected carbohydrates with methyl oxalyl chloride (Scheme 2).4 These esters react with tri-n-butyltin hydride to replace the methyl oxalyloxy group with a hydrogen atom.4–19 Studies of noncarbohydrate esters show that those derived from secondary and tertiary alcohols are suitable starting materials in this deoxygenation process, but esters of primary alcohols are not because they regenerate the alcohols from which they were synthesized.20 Most of the reactions of methyl oxalyl esters of carbohydrates are of compounds in which a tertiary hydroxyl group has been esterified. Many of these compounds are nucleosides.4,7–16 One reason that most methyl oxalyl esters are formed from tertiary alcohols is that the O-thiocarbonyl compounds commonly used for deoxygenation in the Barton-McCombie reaction (Section II in Chapter 12) sometimes have difficulty forming when an alcohol is tertiary.6 Methyl oxalyl chloride typically esterifies tertiary alcohols without difficulty.4,6–19Another reason for selecting methyl oxalyl esters is that they are less likely to experience the thermal elimination (Chugaev reaction) that is common for tertiary O-thiocarbonyl compounds. In molecules with the proper structure cyclization can precede hydrogen-atom abstraction.13
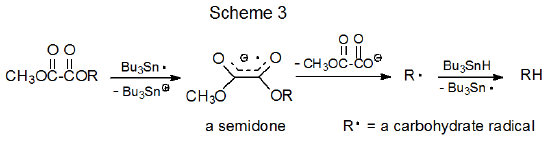
A proposed mechanism for reaction of methyl oxalyl esters with tri-n-butyltin hydride is shown in Scheme 3. According to this mechanism the tri-n-butyltin radical transfers an electron to the π system of the ester to produce a highly stabilized radical anion (a semidione).20 (Supporting the idea that such a transfer takes place is the observation that Bu3Sn· reacts with oxalate esters to produce intermediates with ESR spectra characteristic of radical anions.21) Fragmentation of such a radical anion then generates a carbon-centered radical that abstracts a hydrogen atom from Bu3SnH (Scheme 3).
There are two significant problems associated with the synthesis and reaction of methyl oxalyl esters. One of these is the difficulty in starting- material purification that arises because these esters hydrolyze readily, in particular, during chromatography on silica gel.4,22 A second problem has to do with alcohol regeneration, a significant side reaction from treatment of some methyl oxalyl esters with tri-n-butyltin hydride.5,20
C. Acetates and Trifluoroacetates
Acetylated carbohydrates do not react with tri-n-butyltin hydride under normal conditions (80-110 oC, 2 h, AIBN initiation), but under different, more vigorous conditions (triphenylsilane, 140 oC, 12 h, two equivalents of benzoyl peroxide) these compounds produce the corresponding deoxy sugars (eq 3).23 These more vigorous conditions cause similar reaction in O-trifluoroacetyl substituted carbohydrates.24 The need for two equivalents of benzoyl peroxide in the reaction shown in eq 3 indicates that a nonchain process is taking place.
.png?revision=1&size=bestfit&width=325&height=127)
D. p-Cyanobenzoates
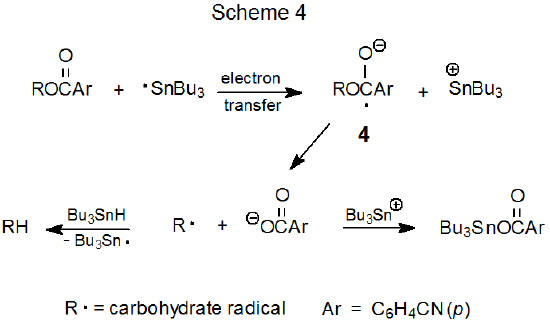
Replacement of the benzoyl group in compound 2 with a p-cyanobenzoyl group converts an unreactive compound (2) into a reactive one (3) (eq 4).25 One explanation for this difference in reactivity is that because a cyano group is quite effective at stabilizing a radical anion, electron transfer to compound 3 is taking place where analogous transfer to the unsubstituted benzoate 2 does not occur. Since radical anions can form by electron transfer from the tri-n-butyltin radical to easily reduced organic compounds,21,26 the electron-transfer mechanism pictured in Scheme 4 represents a possible pathway for replacement of a p-cyanobenzoyloxy group with a hydrogen atom.
.png?revision=1&size=bestfit&width=440&height=163)