III. Radical Cation Formation from Nucleotides
- Page ID
- 24050
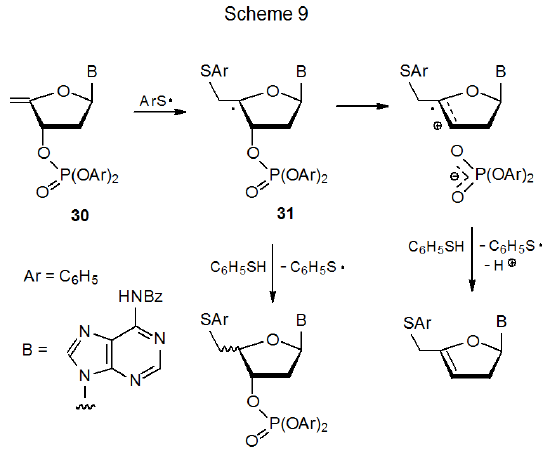
Addition of the phenylthiyl radical to the unsaturated nucleotide 30 produces the carbon-centered radical 31 (Scheme 9).2 This radical (31) either abstracts a hydrogen atom to give an epimeric mixture of reduced nucleotides or fragments the C–O bond at C-3' heterolytically to give a phosphate anion and a radical cation.19 Fragmentation is assisted by polar solvents.20 Since heterolytic fragmentation of 31 also depends on the ability of the substituent at C-3' to stabilize a negative charge as the C–O bond breaks, forming a highly stabilized anion is critical; thus, fragmentation is competitive with hydrogen-atom abstraction when the anion produced is a phosphate (Scheme 9) but not a benzoate (eq 8).2
.png?revision=1&size=bestfit&width=300&height=167)
Additional details concerning ion-pair formation from the unsaturated nucleotide 30 are given in Scheme 10.21–24 A contact ion pair (CIP), a solvent-separated ion pair (SSIP), and diffusively free ions all are included in this Scheme. Labeling experiments show how the various ion pairs participate in the reaction. Since no scrambling of the oxygen label in the phosphate group in the substrate 30 occurs after partial reaction, the CIP either cannot return to the radical 31 or if it does, no reorganization occurs within this ion pair.23 Since oxygen scrambling can take place in the SSIP, the labeling experiments show that once this intermediate is reached, there is no return to the radical 31.
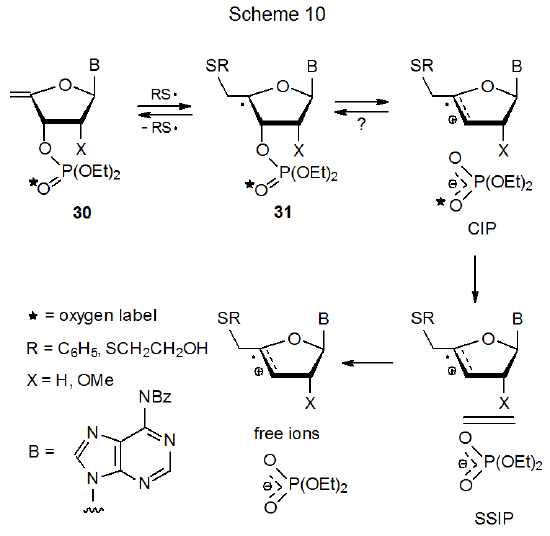
Mechanistic studies using both alkyl- and arylthiols show that the equilibrium between the nucleotide 30 and the adduct radical 31 depends on the identity of the R group in the thiyl radical (Scheme 10). In this equilibrium alkylthiyl radicals favor adduct formation to a greater extent than do arylthiyl radicals. When the method of formation produces a low radical concentration, conditions can exist in which alkylthiyl radicals will form a sufficient concentration of adduct radicals (31) for reaction to proceed at an observable rate, but arylthiyl radicals do not produce the necessary radical concentration.22
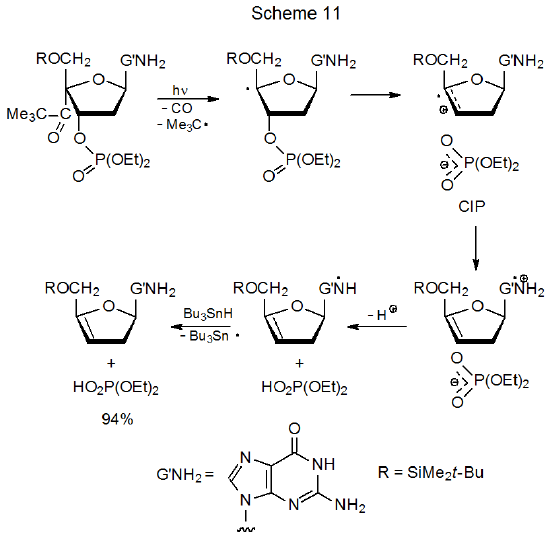
Study of the rates of radical reaction of thymidine, cytidine, adenosine, and guanosine derivatives show that guanosines are by far the most reactive.23 (The rate of reaction of guanosine derivatives is too fast to be measured.) This enhanced reactivity is attributed to rapid, internal electron transfer from the guanine moiety to the radical-cation portion of the molecule (Scheme 11).23,25–27 Electron transfer of this type may be fast enough (k > 1 x 109 s-1) to be taking place within the CIP.23 One estimate of the rate constant for this type of electron transfer is 1.4 x 108 s-1.27