II. Xanthates
- Page ID
- 24064
A. The Carbon Disulfide–Methyl Iodide Procedure (The Standard Procedure for Xanthate Synthesis)
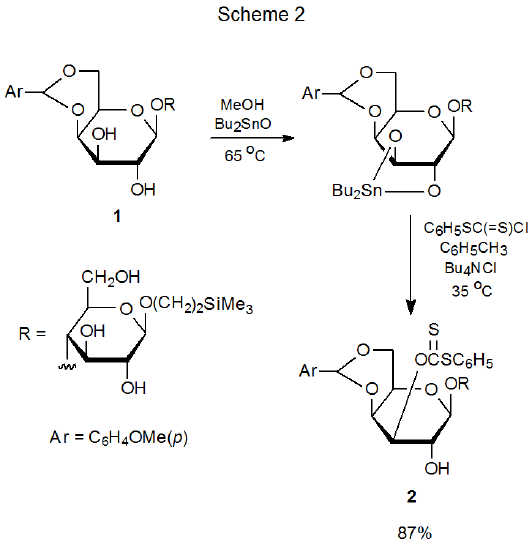
The most common method for synthesizing O-[(alkylthio)thiocarbonyl] esters of carbohydrates (carbohydrate xanthates) begins by forming an alkoxide ion from reaction of sodium hydride with a compound containing an unprotected hydroxyl group.1 (Imidazole usually is present in the reaction mixture to promote alkoxide ion formation.) Once formed, the alkoxide ion adds to carbon disulfide, and the resulting anion is alkylated by methyl iodide. This procedure, which is the standard one for xanthate synthesis, is summarized in Scheme 1.
B. Modifications of the Standard Procedure
1. Reagents and Reaction Conditions
Modification of the procedure outlined in Scheme 1 sometimes is necessary to improve reactant solubility and reactivity. Minor changes take the form of replacing the normal reaction solvent (THF) with N,N-dimethylformamide2,3 or methyl sulfoxide.4–8 When methyl sulfoxide is the reaction solvent, sodium hydroxide usually replaces sodium hydride as the deprotonating base.4,6,7
2. Phase-Transfer Reaction
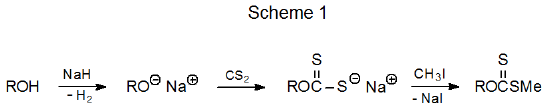
Phase-transfer reaction provides a way for synthesizing xanthates that are difficult or impossible to prepare by the standard procedure. Anomeric xanthates, compounds that provide a synthetic challenge due to their instability, can be produced by phase-transfer reaction (eq 1).9 This reaction also demonstrates the potential of the phase-transfer procedure in preparing xanthates containing base-labile groups.
.png?revision=1&size=bestfit&width=405&height=121)
C. The Phenyl Chlorodithioformate Procedure
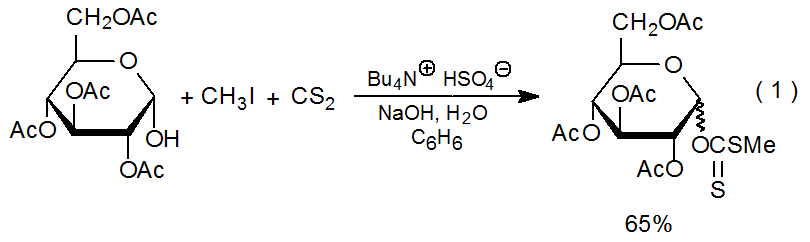
Esterification of an alcohol with an acid chloride provides another, but rarely used procedure for xanthate synthesis. Heating the partially protected disaccharide 1 with dibutyltin oxide produces a stannylene complex that then reacts regioselectively with phenyl chlorodithioformate to give the xanthate 2 (Scheme 2).10 This reaction provides a route to carbohydrate xanthates that contain an O-[(arylthio)thiocarbonyl] group. These arylthio derivatives cannot be prepared by the iodide displacement that is part of the standard xanthate synthesis.