III. (Thiocarbonyl)imidazolides
- Page ID
- 24065
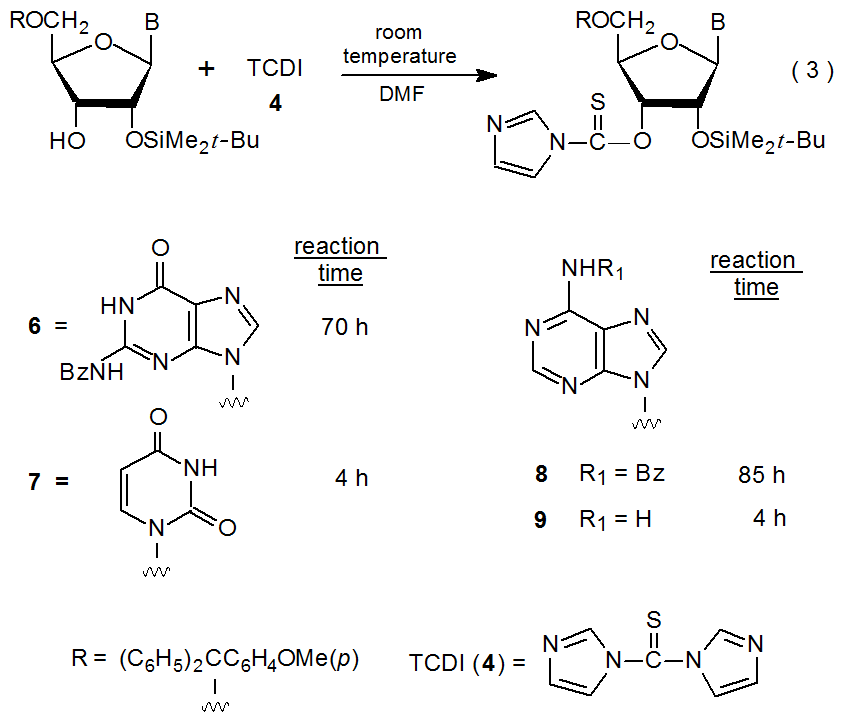
Formation of a (thiocarbonyl)imidazolide (3) generally involves heating a partially protected carbohydrate with N,N-thiocarbonyldiimidazole (4, TCDI) under reflux in tetrahydrofuran (or 1,2-dichloroethane) and isolating the reaction product by chromatography (eq 2).1,11 Nearly every synthesis of a (thiocarbonyl)imidazolide follows this procedure, although acetonitrile,12–14 toluene,15–17 and N,N-dimethylformamide18,19 occasionally are used as reaction solvents.
.png?revision=1&size=bestfit&width=430&height=133)
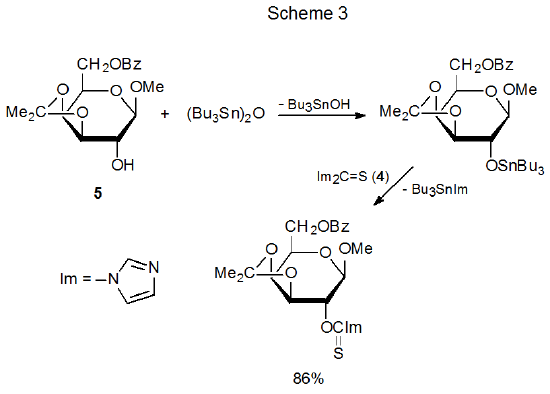
There are scattered reports of (thiocarbonyl)imidazolides forming more slowly than might be expected under typical reaction conditions. One such report concerns the methyl glycoside 5, a compound that reacts so slowly that prior activation with bis(tributyltin)oxide is necessary to increase the nucleophilicity of 5 to the point that (thiocarbonyl)imidazolide formation proceeds at an acceptable rate (Scheme 3).20
Reduced reactivity in nucleosides sometimes is brought about by N‑benzoylation. The N-benzoylguanosine and adenosine derivatives 6 and 8 require treatment with TCDI (4) for 70 and 85 hours, respectively, for complete reaction to take place; in contrast, derivatives lacking the N-benzoyl group (7 and 9), need only four hours for reaction to reach completion (eq 3).21
.png?revision=1&size=bestfit&width=425&height=363)
Even though (thiocarbonyl)imidazolides (3) can be prepared readily by the reaction shown in eq 2, this procedure has several minor drawbacks. One of these is that N,N-thiocarbonyldiimidazole (4, TCDI) needs to be kept in a dry atmosphere because it is unstable in the presence of atmospheric moisture.22 Another is that imidazole, produced as a byproduct in this reaction (eq 2), may catalyze unwanted transformation of some compounds.22 Finally, the cost of TCDI (4) is high enough to be a factor in deciding upon its use, particularly in large-scale reactions.
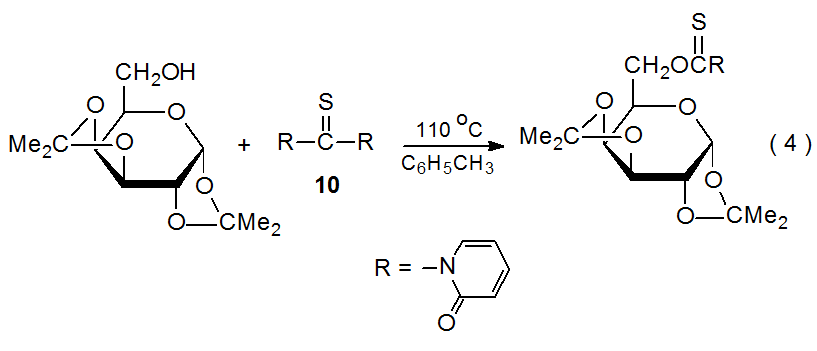
In an effort to overcome possible disadvantages associated with use of (thiocarbonyl)imidazolides, some researchers have proposed switching to related compounds. Thionocarbamates formed from 1,1'-thiocarbonyldi-2,2'-pyridone (10), a reagent stable to atmospheric moisture, are effective replacements for (thiocarbonyl)imidazolides (eq 4),22 but detracting from the use of this new reagent (10) is its even greater cost that TCDI.
.png?revision=1&size=bestfit&width=410&height=176)
Some thionocarbamates synthesized from the inexpensive phenyl isothiocyanate (11) (eq 5) are capable of radical formation.23,24 Although producing a thionocarbamate by reacting a partially protected carbohydrate with phenyl isothiocyanate (11) solves the “cost problem”, it has the disadvantage that this reaction requires the presence of a strong base because hydroxyl group deprotonation is needed for this reaction to occur at an acceptable rate (eq 5). Also, not all thionocarbamates prepared from 11 form radicals under typical reaction conditions.25 None of the alternatives to (thiocarbonyl)imidazolides have been widely adopted.
.png?revision=1&size=bestfit&width=430&height=194)

