IV. Aryl Thionocarbonates
- Page ID
- 24066
A. Reaction with Phenoxythiocarbonyl Chloride
1. DMAP-Catalyzed Reactions
The standard procedure for synthesis of phenyl thionocarbonates is illustrated by the reaction shown in eq 6.26,27 Phenoxythiocarbonyl chloride (12) in the presence of the powerful, acylation catalyst 4-dimethylaminopyridine (13, DMAP) esterifies most carbohydrates with ease. Even though pyridine itself can be used in some situations, the superior catalytic effect of DMAP makes it the reagent of choice. In those rare instances when thionocarbonate formation by the standard procedure is too slow, switching the reaction solvent from acetonitrile to N,N-dimethylformamide or methyl sulfoxide often is sufficient to increase the rate of reaction to a synthetically acceptable level.28
.png?revision=1&size=bestfit&width=430&height=196)
DMAP (13) causes acylation rates to increase by factors as large as 10,000 when compared to reactions catalyzed by pyridine.29 One possibility for the greater catalytic effect of DMAP is that it is a stronger base than pyridine. (The pKb for pyridine is 8.71 and that for DMAP is 4.30.29) This explanation for the difference in reactivity, however, is not sufficient to explain DMAP’s superior catalytic ability because triethylamine (pKb = 3.35), an even stronger base than DMAP, has a catalytic effect similar to that of pyridine.29
A better explanation for DMAP being such an effective catalyst is that it reacts with acid chlorides, such as 12, to form high concentrations of N‑acylpyridinium salts (eq 7).29 These salts are better able to transfer an acyl group to a nucleophile than is the acid chloride itself. Resonance stabilization (two of the principal resonance contributors are shown in eq 7) increases the equilibrium concentration of an N-acylpyridinium salt, and charge delocalization increases the reactivity of this powerful acylating agent by creating loosely bound ion pairs.
.png?revision=1&size=bestfit&width=430&height=154)
The mildly basic conditions for thionocarbonate synthesis stand in contrast to the strongly basic ones used for xanthate preparation.26 Avoiding strongly basic conditions often is necessary in nucleoside synthesis; for example, thionocarbonates such as 14 can be prepared without difficulty (by the procedure outlined in eq 6), but attempted synthesis of the corresponding xanthates results in starting material decomposition.27 The specific reason xanthate synthesis fails in this case is that it requires conditions too basic for the stability of nucleosides protected by the 1,1,3,3-tetraisopropyl-1,3-disiloxanediyl group, a common protecting group for nucleosides.
2. N-Hydroxysuccinimide-Catalyzed Reactions
Although DMAP (13) is the catalyst of choice in most syntheses of phenyl thionocarbonates, sometimes, in an effort to avoid an undesired, competing reaction or to improve product yield, DMAP is replaced by another reagent. The most common replacement is N-hydroxysuccinimide (NHS, 15).30–35 In the reaction shown eq 8, the methyl pyranoside 16 gives a better yield of the corresponding phenoxythionocarbonate when NHS (15) is the catalyst rather than DMAP.16
.png?revision=1&size=bestfit&width=425&height=185)
There is a similarity in the mode of action of DMAP and NHS in that each of them typically reacts with an acid chloride to produce a better acylating agent. For DMAP (13) this agent is the N-acylpyridinium salt shown in eq 7, and for NHS (15) the new acylating agent is the ester 17 (eq 9). Because esters of NHS react unusually rapidly with nucleophiles, they are sometimes referred to as "activated esters".36
.png?revision=1&size=bestfit&width=420&height=126)
Extensive mechanistic study of esters of N-hydroxysuccinimide with amines has shown their reaction kinetics to be consistent with a process in which reversible formation of the zwitterionic intermediate 18 is followed by a rate-determining breakdown of this intermediate by either an uncatalyzed or base-catalyzed process (Scheme 4).37,38 Since the hydroxyl group in NHS (15) is quite acidic (pKa = 6.039), its conjugate base (19) is more stable than most alkoxide ions. To the extent that the stability of the departing anion contributes to transition state stabilization (Scheme 4), esters derived from NHS should be particularly reactive.
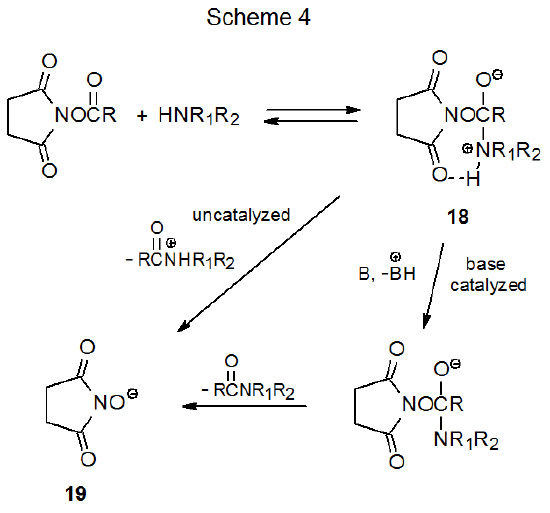
The mechanism shown in Scheme 5 is based on the assumption that the findings from amine acylation (Scheme 4) can be extended to thioacylation of carbohydrates. Base-catalyzed reaction seems most reasonable, but the mechanism shown in Scheme 5 also includes an uncatalyzed process in which the initially formed, tetrahedral intermediate 20 undergoes proton transfer to give the zwitterion 21. This intermediate eliminates separation of charge by expelling a tautomer of NHS to form the desired phenyl thionocarbonate 22.
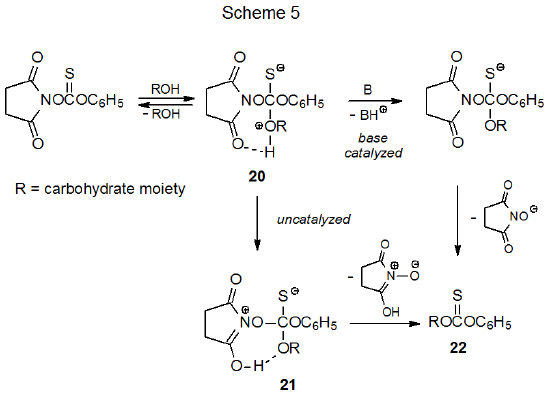
B. Reaction with Thiophosgene and a Phenol
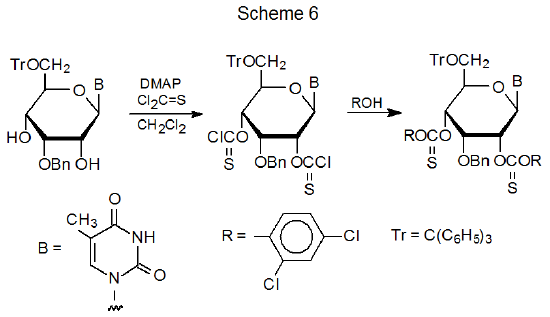
An alternative synthesis for an aryl thionocarbonate consists of treating a partially protected carbohydrate with thiophosgene and then reacting the product with a phenol (Scheme 6).40–47 Since phenoxythiocarbonyl chloride (12) is commercially available, the thiophosgene procedure normally is reserved for preparing aryl thionocarbonates in which the aromatic ring contains one or more electron-withdrawing substituents. In direct reactivity comparisons, substituted aryl thionocarbonates usually give better product yields.40 In some cases these substituents are necessary for reaction to take place.41
C. Reaction of (Thiocarbonyl)imidazolides with Phenols
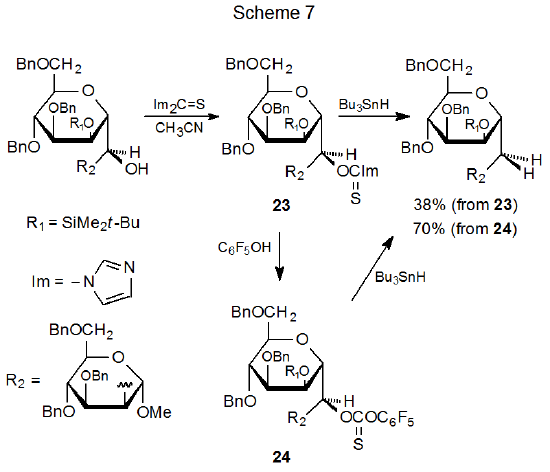
Thionocarbonates are sometimes synthesized by reacting (thiocarbonyl)imidazolides with a substituted phenol. Such a reaction converts a less reactive O-thiocarbonyl derivative into a more reactive one (Scheme 7).48 It also provides another method for synthesizing aryl thionocarbonates in which the aromatic ring contains one or more electron-withdrawing substituents. Affecting the change shown in Scheme 7 causes the deoxygenated product yield to rise from 38% (starting with 23) to 70% (starting with 24).
D. Reaction With Phenoxythiocarbonyltetrazole
The thioacylating agent 25 can be used to synthesize phenyl thionocarbonates under conditions that avoid the base-catalyzed side reactions that sometimes occur in the presence of DMAP (eq 10).49
.png?revision=1&size=bestfit&width=425&height=108)

