VI. Thionoesters
- Page ID
- 24068
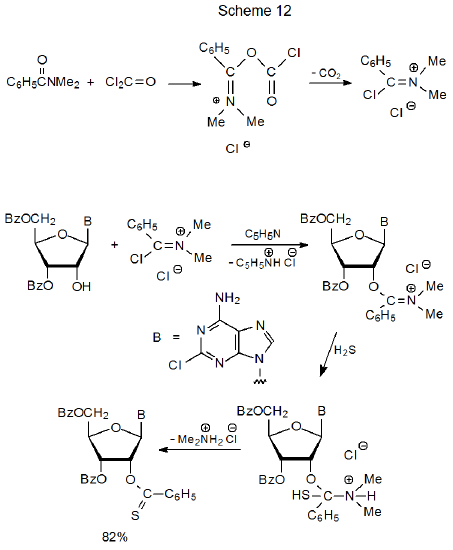
The standard synthesis of thionoesters is shown in eq 14. Scheme 12, which contains a more detailed picture of this sequence, includes a proposed mechanism for this reaction.1 Although this method of thionoester preparation is effective, it requires handling the toxic gases phosgene and hydrogen sulfide.27 This added difficulty in preparation is a factor in thionoesters being used less frequently than other, O-thiocarbonyl carbohydrate derivatives.
.png?revision=1&size=bestfit&width=425&height=192)
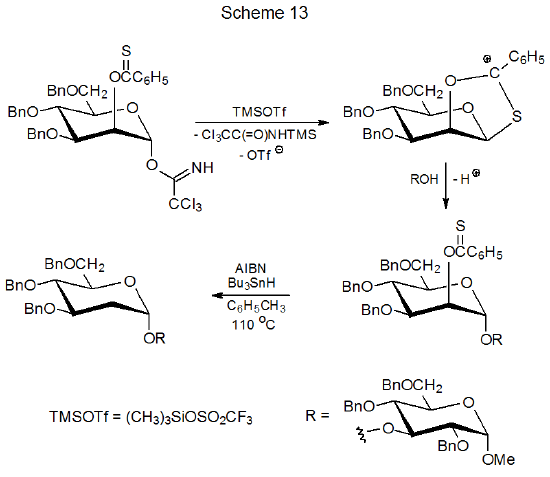
Thionobenzoates are used for radical formation more often than other thionoesters. Although conditions for preparation of thionobenzoates make them less attractive starting materials that other O-thiocarbonyl compounds, these esters become more desirable reactants if the O-thiobenzoyl group has an additional role in the reaction. In the transformation shown in Scheme 13 the 2-O-thiobenzoyl group anchimerically assists glycoside formation prior to participating in radical reaction.60,61.