IV. Addition Reactions
- Page ID
- 24080
A. A Competition Always Present
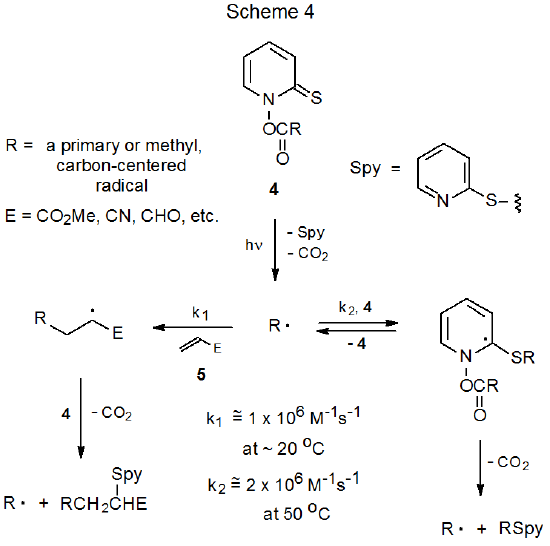
The rate constant for addition of a typical carbon-centered radical to an ester of N-hydroxypyridine-2-thione (kr \(\cong\) 2 x 106 M-1s-1 at 50 oC)29,30 is large enough that competing addition of such a radical to a reactant with a carbon–carbon multiple bond occurs only for compounds with the most reactive bonds (i.e., those with electron-withdrawing substituents attached.) (The rate constants for addition to these unsaturated reactants are approximately 1 x 106 M-1s-1 at 20 oC31) In practice this means that unless the multiple bond has an electron-withdrawing substituent, the rate of addition of a carbon-centered radical will be too slow to compete effectively with addition to a Barton ester. As is emphasized in Scheme 4, a similarity in rate constants means that the competition between addition of a radical to the unsaturated compound 5, as opposed to addition to a second molecule of the Barton ester 4, depends heavily upon the relative concentrations of these two reactants (4 and 5).
Examples of the competition between radical addition to a molecule with an electron deficient double bond or to a molecule of unreacted starting ester are found in the reactions shown in equations 4-7. In the first of these (eq 4) acrylamide is present in two-fold excess; yet, products from addition of R· to the amide and to the starting ester are formed in essentially equal amounts.32 In the second reaction (eq 5) a good yield of the product from radical addition to methyl acrylate requires a five-fold excess of the unsaturated ester.33 In the reaction shown in eq 6 even a six-fold excess of phenyl vinyl sulfone does not suppress completely addition to the starting ester of some of the carbohydrate radicals.34 Radical addition to 2‑nitropropene is similar to addition to other unsaturated compounds (eq 7).35,36
.png?revision=1&size=bestfit&width=410&height=164)
.png?revision=1&size=bestfit&width=420&height=190)
.png?revision=1&size=bestfit&width=415&height=177)
.png?revision=1&size=bestfit&width=420&height=214)
B. Compounds With Electron-Deficient Multiple Bonds
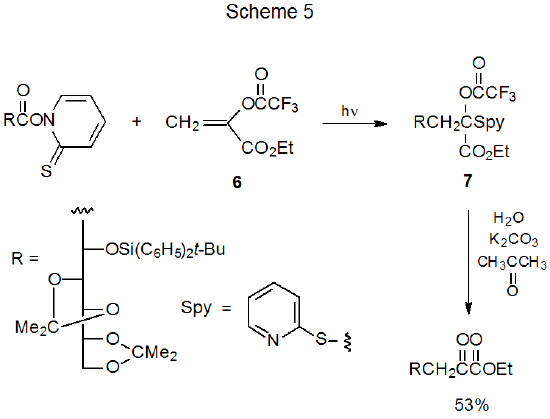
Generalizing the information in equations 4-7 leads to the conclusion that carbohydrate radicals formed from esters of N-hydroxypyridine-2-thione add to compounds with electron- deficient multiple bonds; specifically, these reactions include radicals combining with α,β-unsaturated amides32,37,38 and esters33,35,39–41 as well as with compounds in which a double bond has either a phenylsulfonyl 34,39,42 or nitro35,36 substituent. Among reactive compounds addition to the carbon–carbon double bond in ethyl α‑(trifluoroacetoxy)acrylate (6)32,35,43–47 is reported more often than addition to any other compound (Scheme 5). One reason for this is that the resulting adduct (7) hydrolyzes under very mild conditions to give an α‑keto ester; thus, radical addition extends the carbon chain by two atoms and introduces easily manipulated functional groups (Scheme 5).47
C. Heteroaromatic Compounds
As the reaction in eq 8 shows, radicals generated from N-hydroxypyridine-2-thione esters also add to aromatic amines.34,42,48,49 Since this type of reaction occurs only when an acid such at trifluoroacetic acid is present in the reaction mixture, the assumption is that the radical is adding to the protonated amine. [In the absence of an acid only RSPy (eq 8) is formed.] Such an assumption is reasonable because a nucleophilic radical would be expected to react more rapidly with an electron-deficient π system.
.png?revision=1&size=bestfit&width=385&height=202)
D. Carbohydrates as Chiral Auxiliaries
One synthetic application of carbohydrate-containing esters of N‑hydroxypyridine-2-thione uses the carbohydrate moiety as a chiral auxiliary during radical addition.35,36,40,41,43 The reaction shown in eq 9 illustrates the diastereoselectivity possible in this type of process.35 Selectivity in this case results primarily from steric interactions between methyl acrylate and the C-6 substituent in the reactant sugar.35
.png?revision=1&size=bestfit&width=430&height=233)

