II. Reaction Mechanisms
- Page ID
- 24085
A. Addition-Elimination Reaction
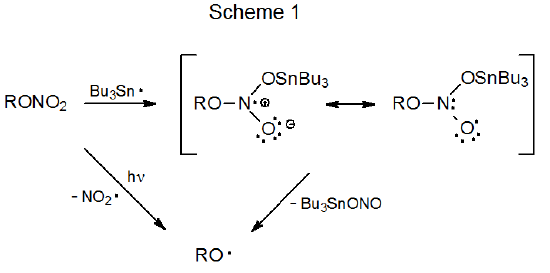
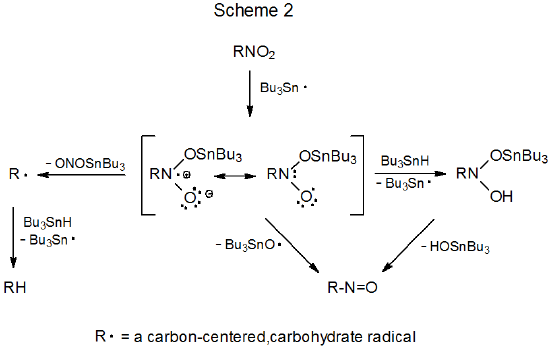
The first step in the reaction of the tri-n-butyltin radical with a nitro compound is the addition of the radical to one of the oxygen atoms in the nitro group. The reaction that takes place after this initial addition depends upon whether the reactant is an O-nitro or a C-nitro compound. O‑Nitro carbohydrates fragment to give alkoxy radicals (Scheme 1). C-Nitro carbohydrates have more varied possibilities. The adduct radical either breaks the C–N bond to give a carbon-centered radical, cleaves an O–N bond to form a nitroso compound, or abstracts a hydrogen atom from an available donor, usually Bu3SnH (Scheme 2).
B. Electron Transfer
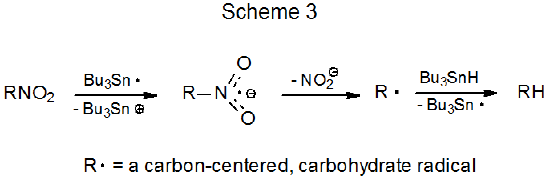
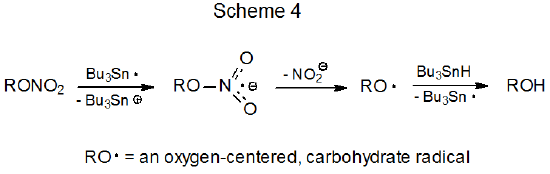
Early investigations raised the possibility that reaction of a C-nitro compound with the tri-n-butyltin radical could involve electron transfer (Scheme 3).1–3 Later investigation, however, did not support this possibility because the electron transfer between Bu3Sn· and (CH3)2CHNO2 (Scheme 3) was found to be endothermic by at least 12 kcal mol-1 . This endothermic electron transfer was inconsistent with the large rate constant (k = 9.5 x 107 M-1s-1) observed for reaction between this pair [Bu3Sn· and (CH3)2CHNO2].4 The conclusion from this latter study was that the addition-elimination mechanism (Scheme 2)5–8 provided a better explanation for the reaction between Bu3Sn· and a C‑nitro compound. The possibility that electron transfer could be involved in reaction of O-nitro compounds (Scheme 4) has not been addressed and, thus, remains open.
C. Photochemical Reaction
Photolysis of an O-nitrocarbohydrate fragments the N–O bond in the nitro group to produce nitrogen dioxide and the corresponding alkoxy radical (Scheme 1).

