IV. Nonchain Reactions: Radical Formation by Electron Transfer
- Page ID
- 24616
A. Transition-Metal Complexes as Electron Donors
As is indicated in Scheme 3, radical formation in a nonchain reaction often occurs by dissociative electron transfer from a transition-metal complex (e.g., Cp2TiCl) to a halogenated carbohydrate. An example of such a reaction is shown in eq 38, where electron donation by Cp2TiCl enables a pyranos-1-yl radical to be formed from the glycosyl bromide 1; this radical then adds to methyl vinyl ketone.181 Other reactions of this type, all of which are nonchain and involve pyranos-1-yl radicals generated from glycosyl bromides, are listed in Table 6.
.png?revision=1&size=bestfit&width=420&height=111)
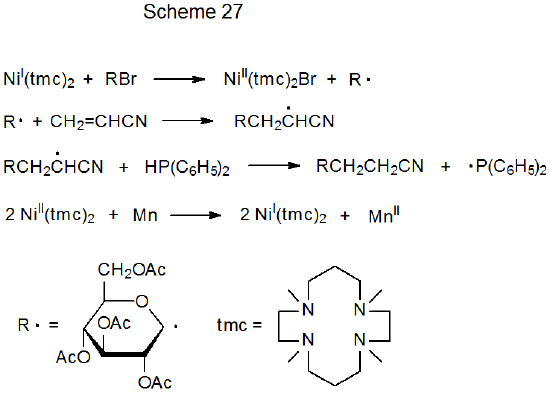
Although the reactions shown in Table 6 are nonchain in nature and, thus, do not require the typical addition of a catalytic amount of an initiator, they can be made catalytic in the transition-metal complex, if the metal ion in the complex is returned to its original oxidation state quickly after electron transfer. An example of a reaction taking place by such a process is found in Scheme 27, where Ni(I) is proposed to be continuously formed by reaction of Ni(II) with manganese metal.25
Cobalt is another transition metal capable of forming carbohydrate radicals by electron transfer.183–187 An overall reaction showing electron donation by a cobalt complex is given in eq 39, but the transfer actually takes place in two, distinct steps (equations 40 and 41). Because many cobalt complexes of carbohydrates are stable enough to be isolated, the radical forming step for reactions of such compounds is carbon–cobalt bond homolysis (eq 41). Eq 42 describes a reaction in which a pyranos-1-yl radical, produced by C–Co bond homolysis, adds to a molecule of styrene.184
.png?revision=1&size=bestfit&width=410&height=94)
.png?revision=1&size=bestfit&width=440&height=79)
B. Transition-Metal Complexes as Electron Acceptors
Reaction of a CH-acidic compound such as CH2(CO2CH3)2 with (NH4)2Ce(NO3)6 [or Mn(OAc)3] transfers an electron to the transition-metal complex to produce ·CH(CO2CH3)2, an electron-deficient radical (Scheme 4).188–192 This radical then adds to an electron-rich double bond, such as that found in a typical glycal (eq 43).189 A similar glycal addition takes place with the electrophilic radical CH3NO2·, which is formed by electron transfer from CH3NO2- to (NH4)2Ce(NO3)6.193
.png?revision=1&size=bestfit&width=420&height=200)

