V. An Organization for Carbohydrates That Undergo Radical Cyclization
- Page ID
- 24622
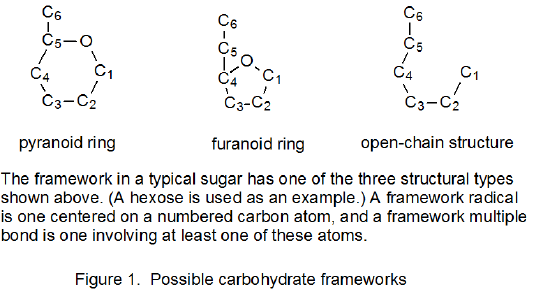
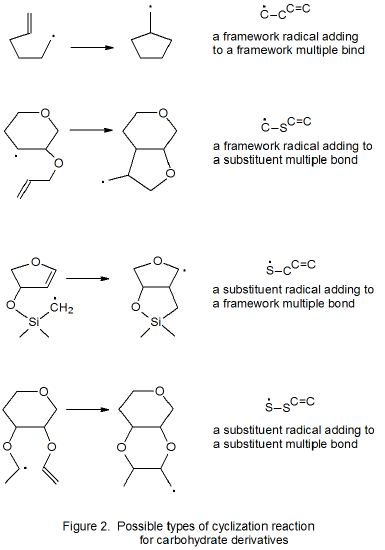
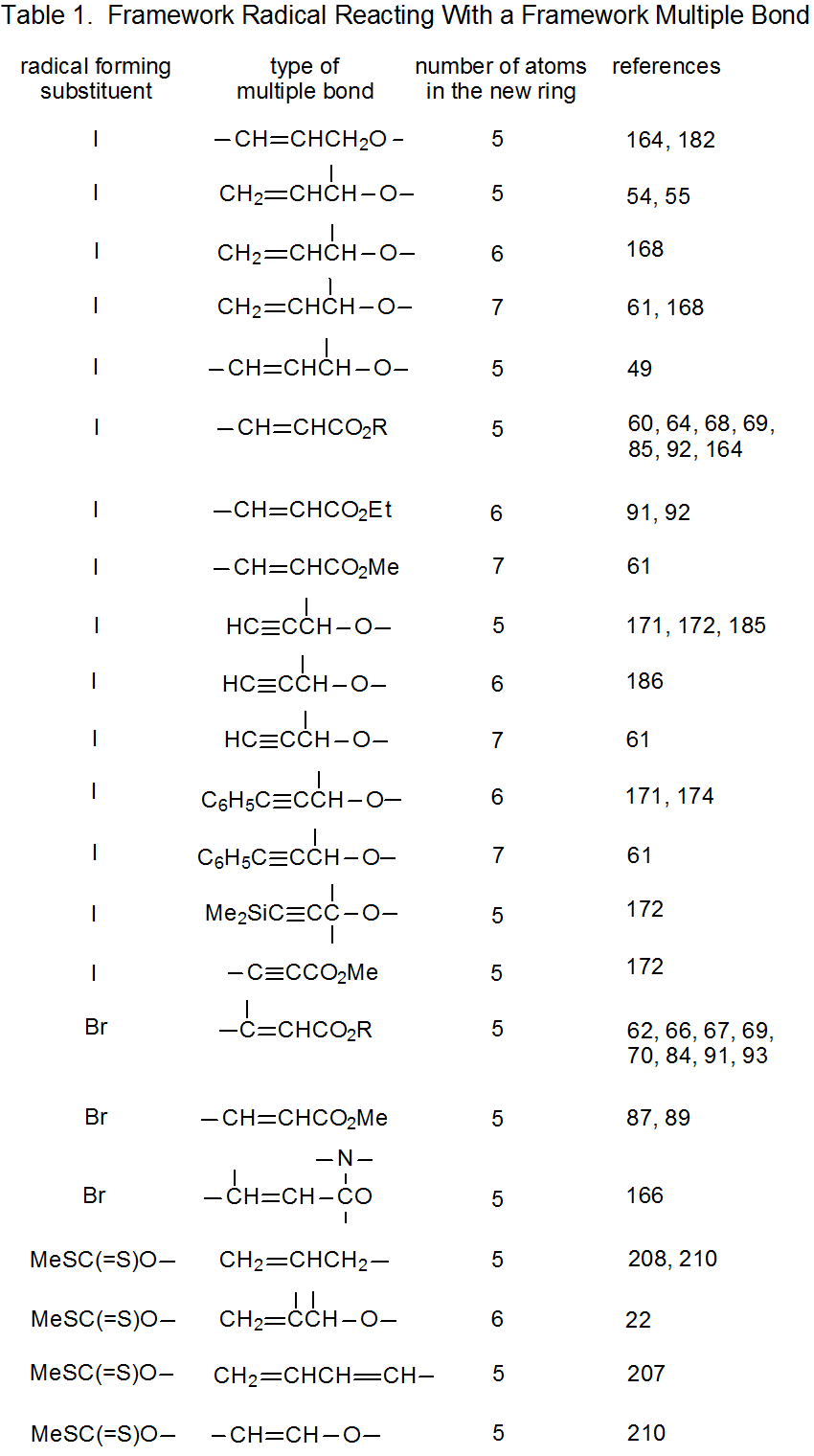
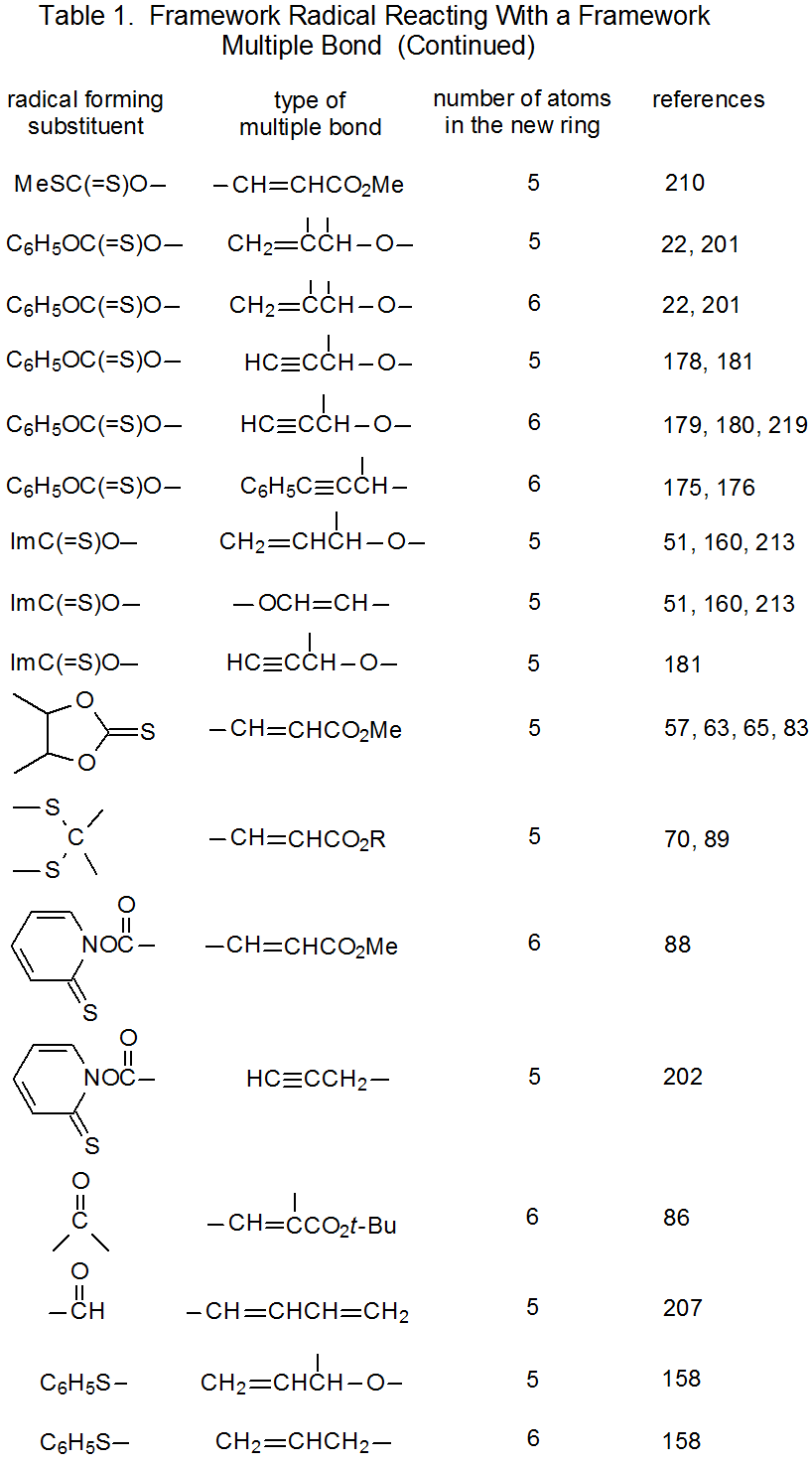
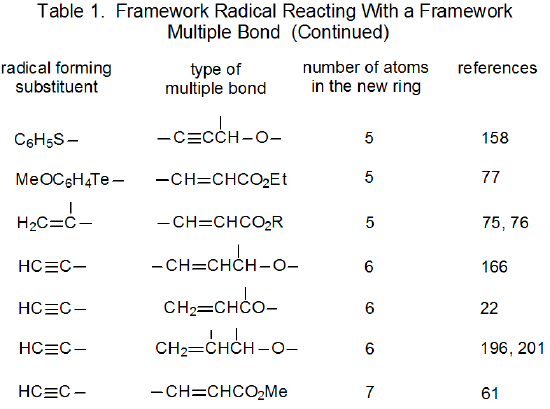
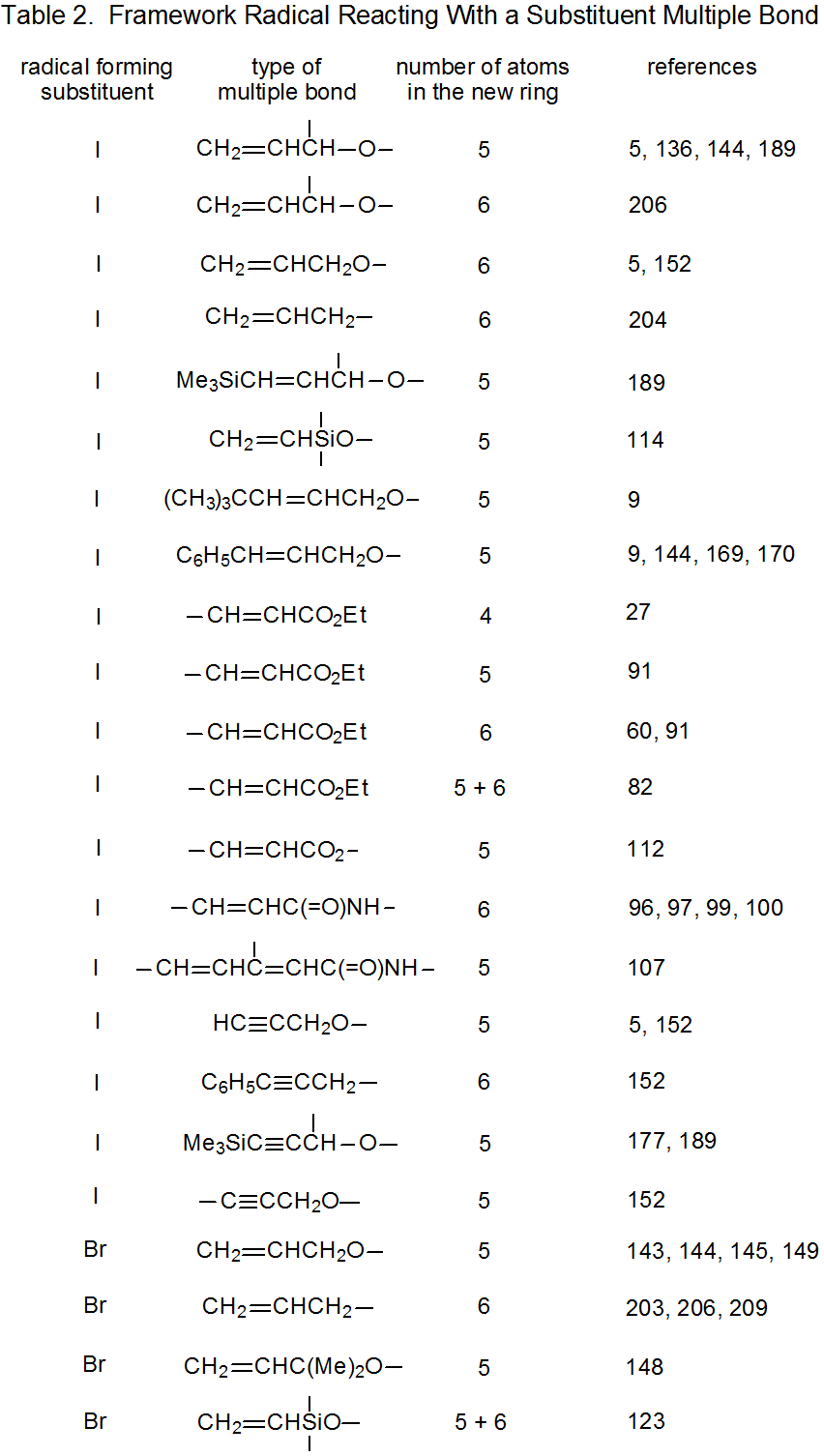
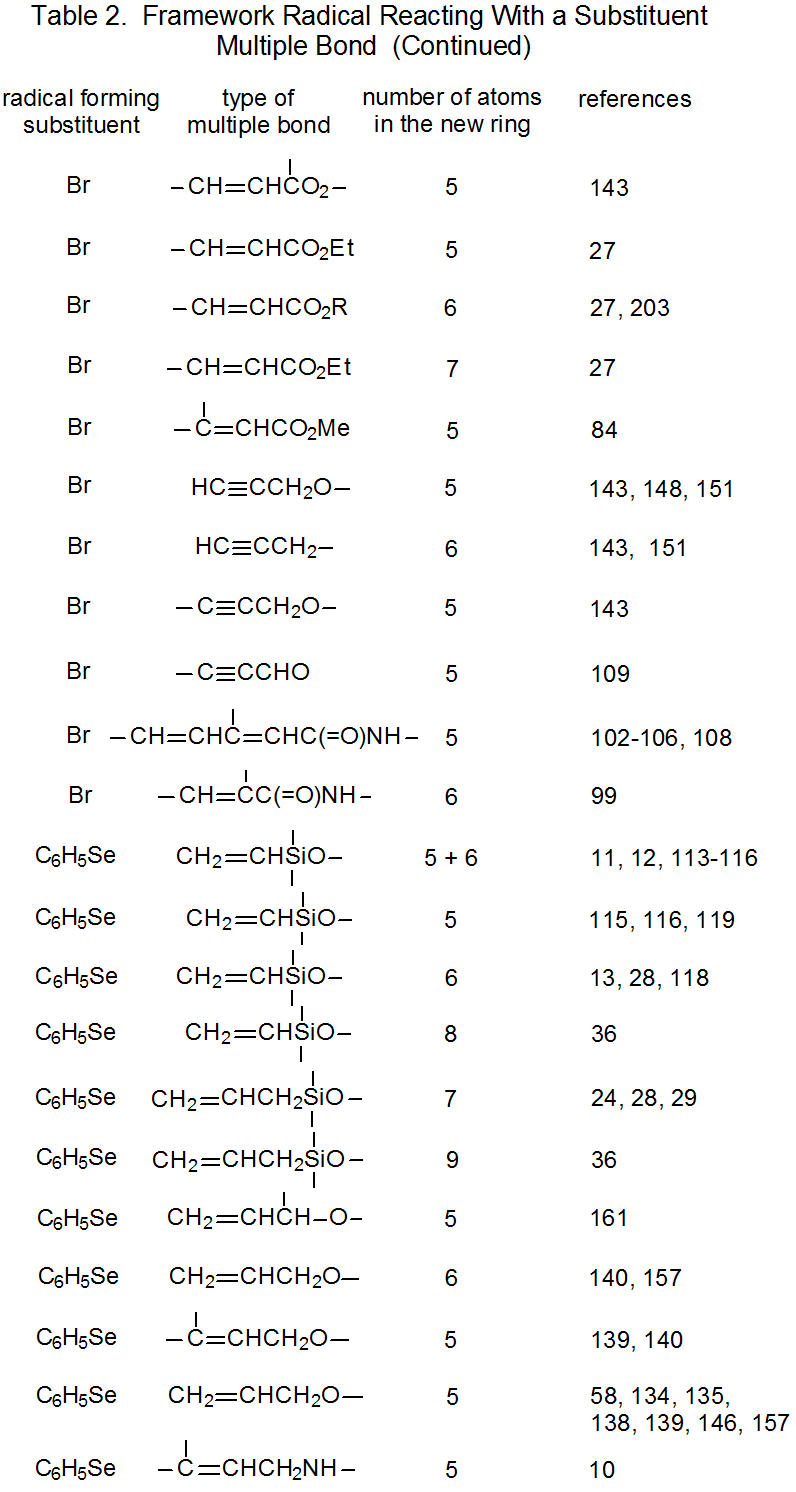
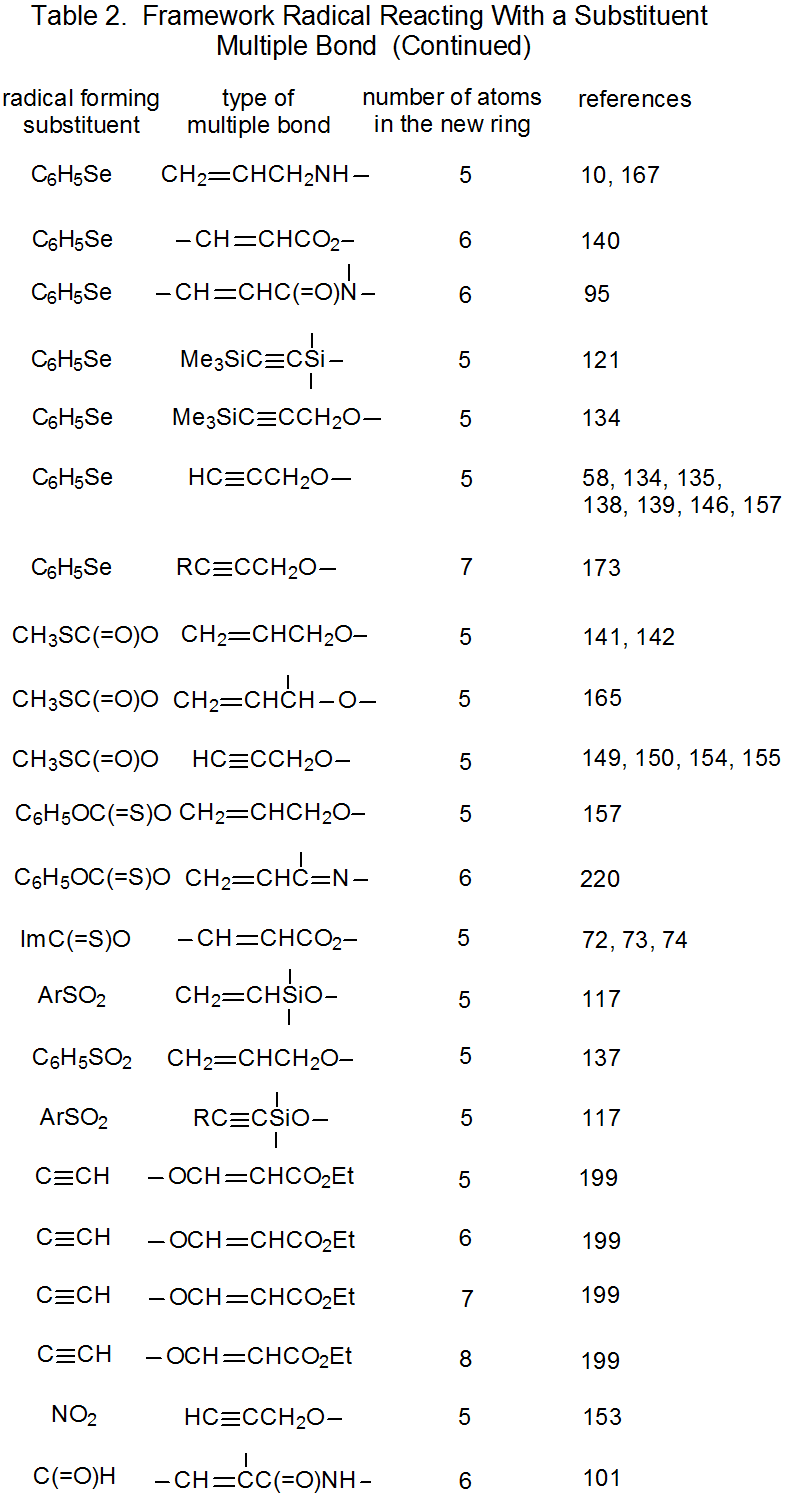
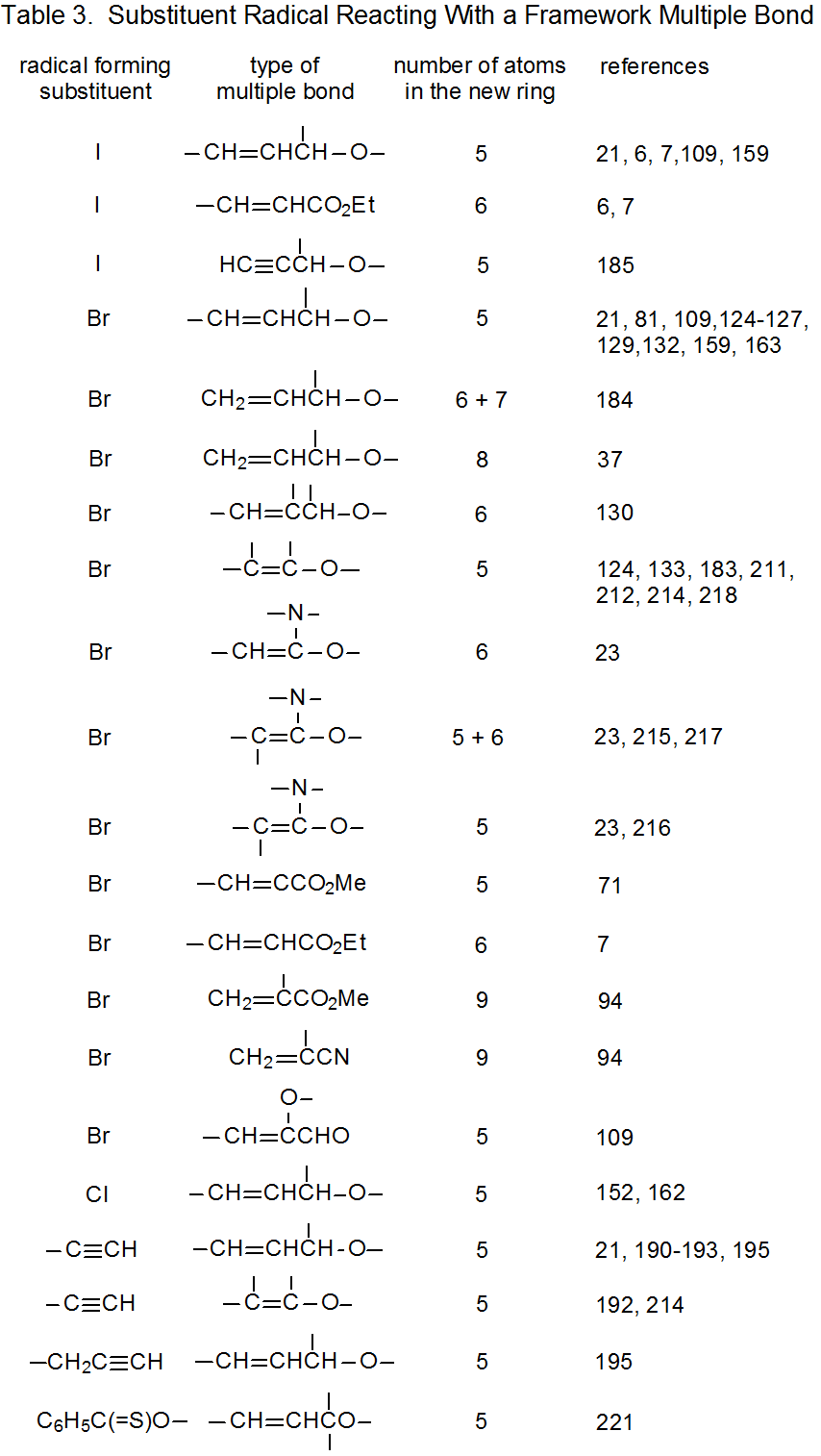
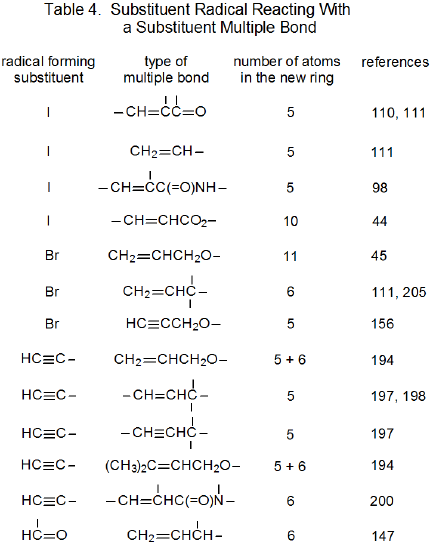
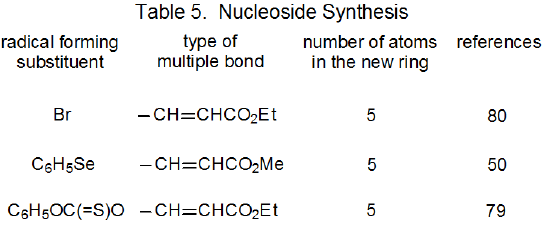
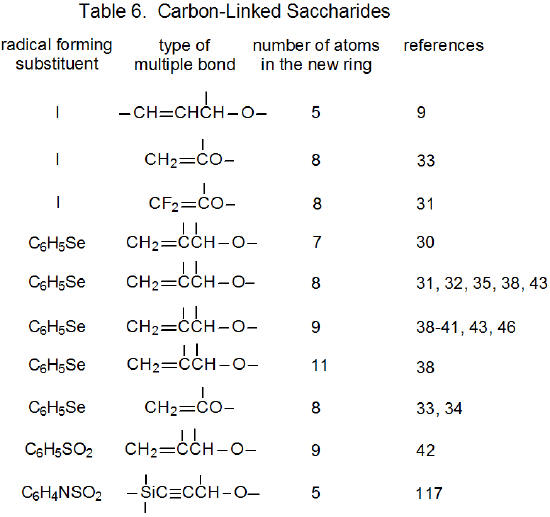
It is useful in organizing radical cyclization reactions to divide them into groups that have common features. One method for doing this places radicals of similar structure together. Where carbohydrates are concerned, such a plan can be based on the location of the radical center and the multiple bond. A radical center can exist on an atom that is part of the molecular framework (Figure 1) or part of a substituent group. The same possibilities exist for the multiple bond. Cyclization reactions of carbohydrates then naturally divide into the four basic types shown in Figure 2. (A short-hand terminology describing these four types has been proposed45 and is included in Figure 2.) This division provides the basis for constructing Tables 1-4. In addition to these four tables, two smaller ones are included in recognition of the importance of radical cyclization reactions in the synthesis of nucleosides (Table 5) and carbon-linked disaccharides (Table 6.)