IV. Reactions of Organosamarium Compounds
- Page ID
- 24627
A. Protonation
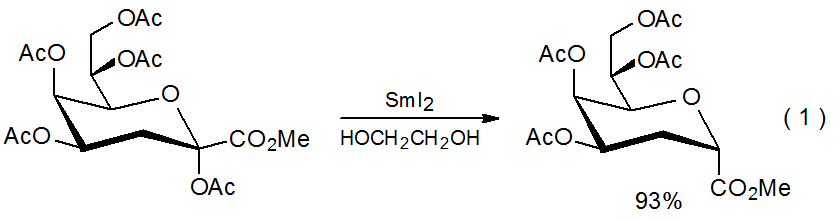
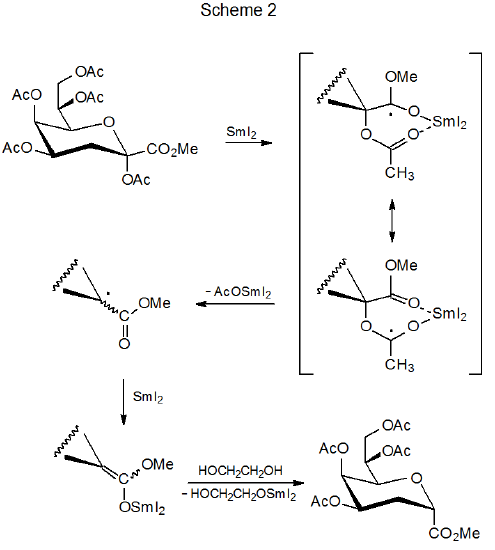
Accepting a proton from a suitable donor is a characteristic reaction of an organosamarium compound.19–28 Lactones with α substituents19–23 and esters that are similarly substituted22,25 are common substrates in this type of reaction. Equation 1 describes a typical example.22 A mechanism for the reaction shown in eq 1 is proposed in Scheme 2. Tosylates26 and sulfones27 also form organosamarium compounds that readily protonate.
.png?revision=1&size=bestfit&width=420&height=110)
B. β Elimination
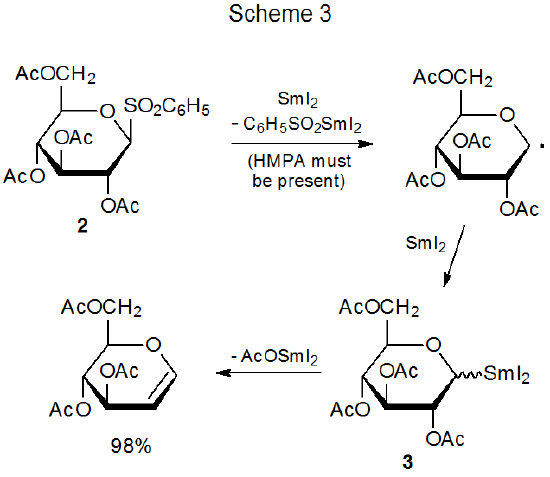
Another characteristic reaction of organosamarium compounds is eliminating a samarium-containing group along with a substituent on a neighboring carbon atom to form a compound with a carbon–carbon double bond.19,20,27,29–32 An example of a reaction in which this happens is shown in Scheme 3, where the glycosyl phenyl sulfone 2 reacts with SmI2 to give the organosamarium intermediate 3, from which β elimination produces the corresponding glycal.27
In the reactions of glycosyl phenyl sulfones with SmI2 the amount of glycal formed depends upon how well the departing, C-2 substituent supports a negative charge. In the reaction shown in eq 2 the compound with the O‑acetyl group at C-2 gives a far higher yield of glycal than does the substrate with the O-benzyl group in the 2-position.27,32 The primary process competing with glycal formation in this reaction is proton transfer to the organosamarium intermediate from the trace amount of water present in the reaction mixture. For the substrate with the O-acetyl group, proton transfer from water is too slow to be of consequence, but for that with the O‑benzyl group proton transfer is significant and becomes the major reaction pathway when greater than trace amounts of water are present (eq 2).
.png?revision=1&size=bestfit&width=405&height=211)
C. Addition to a Carbonyl Compound
Scheme 4 describes a reaction between samarium(II) iodide and a carbohydrate with an arylsulfonyl group to give an organosamarium compound that then adds to cyclohexanone.33 Addition to aldehydes and ketones is another characteristic reaction of an organosamarium intermediate. In Scheme 4 the overall reaction is given first, and then a mechanism for the radical and nonradical phases of the reaction is proposed.
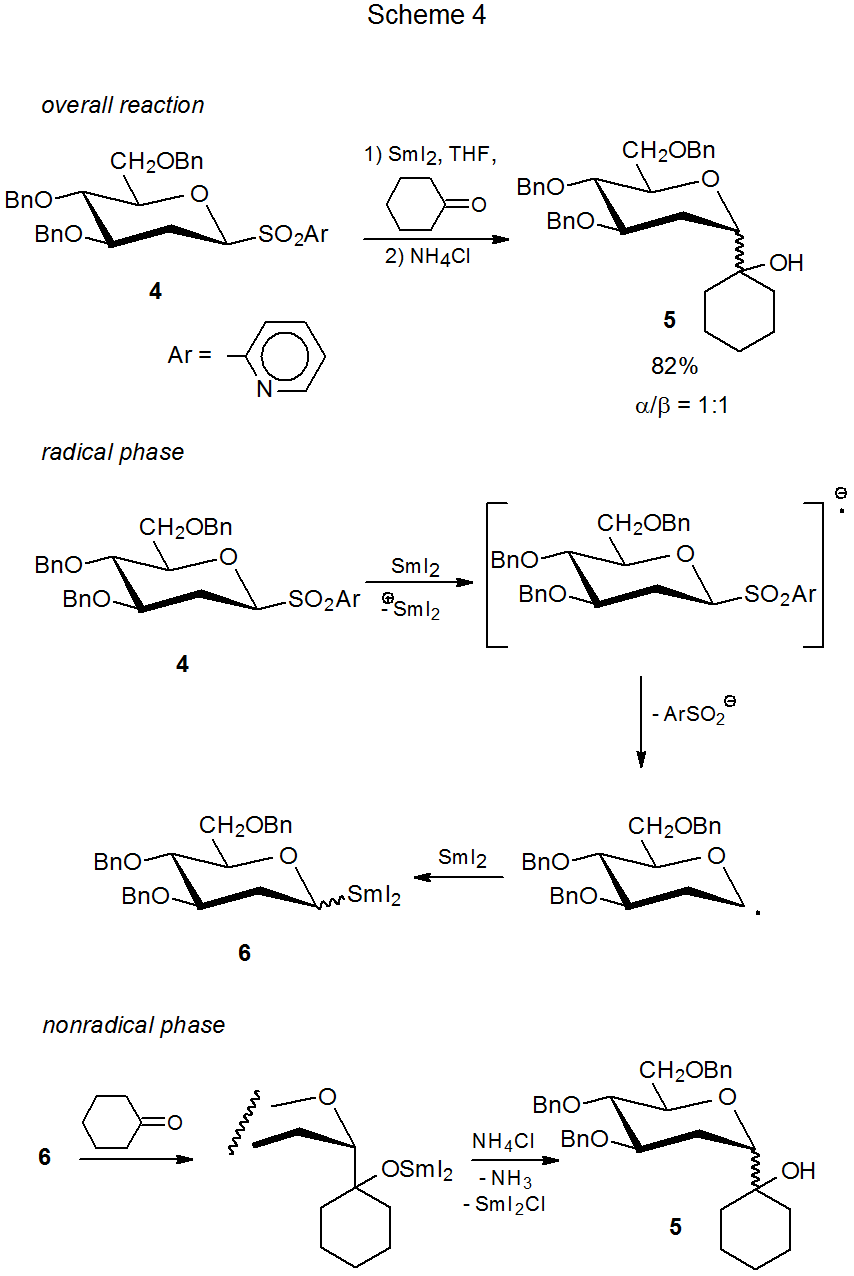
1. The Samarium-Barbier Reaction
Because in the reaction shown in Scheme 4 the sulfone 4 and cyclohexanone both are present in the reaction mixture from the outset, the reaction is described as a Barbier-type2 or samarium-Barbier3,34 reaction. The mechanism pictured in Scheme 4 is a widely accepted one for this type of process.1–9,34–37 The carbohydrate reactant frequently is a glycosyl sulfone,28‑30,32,33,37‑43 but it also can be a glycosyl halide31,32,37,44-46 or phosphate.47 Possibilities for the carbonyl compound include ketones,2,28,32,39,42,43,46 aldehydes,2,28–30,32,38–46, and lactones.45, 48–50 Usually the carbonyl compound is a simple organic molecule, but sometimes the carbonyl group is part of the more complex structure found in a carbohydrate.38–40,42–44
2. The Samarium-Grignard Reaction
The defining characteristic of the samarium-Barbier reaction is that all of the reactants are present in the reaction mixture at the outset. If an intermediate organosamarium compound is sufficiently stable, it can be formed prior to adding the carbonyl compound. When reaction takes place using such a procedure, it is described as a Grignard-type or samarium-Grignard reaction.3,34 Many organosamarium compounds are not stable enough to undergo reaction in this way; in particular, the reaction shown in Scheme 4 is only successful when run under samarium-Barbier conditions.33
D. Formation and Reaction of Samarium Ketyls
Reaction of samarium(II) iodide with aldehydes and ketones produces ketyl radical anions, sometimes referred to as samarium ketyls (eq 3). These intermediates, each of which has considerable radical character on the former carbonyl carbon atom, form reversibly and have longer lifetimes than typical radical anions and most carbon-centered radicals.
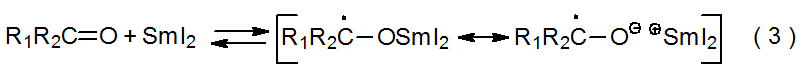.png?revision=1&size=bestfit&width=405&height=39)
1. Internal Addition to a Carbon–Carbon Multiple Bond
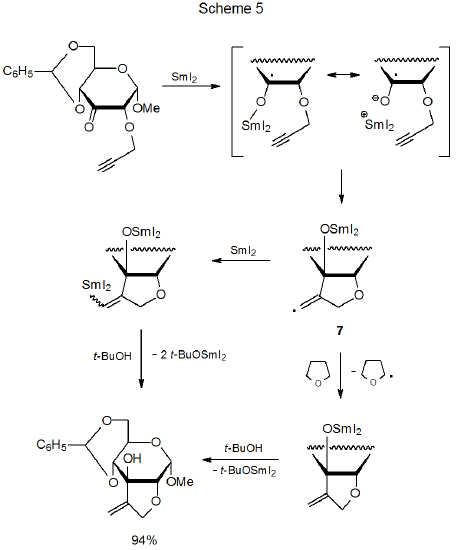
A samarium ketyl that contains a properly positioned multiple bond readily forms a new ring system.51–62 Examples indicating the range of reactivity of these ketyl intermediates are found in the reactions shown in equations 4 and 5 and Scheme 5. In the reaction pictured in eq 4, an unsaturated aldehyde forms a samarium ketyl that cyclizes and then reacts with cyclohexanone.60 Eq 5 describes the reaction of an unsaturated carbonyl compound that has a substituent on the carbon atom α to the carbonyl group. If the substituent is a poor leaving group [e.g., (C6H5)3CO], ring formation takes place, but when a better leaving group [e.g., (CH3)3CCO2] is present, cyclization is replaced by elimination of the corresponding anion [e.g., (CH3)3CCO2-] followed by hydrogen-atom abstraction.62 The highly stereoselective cyclization shown in Scheme 5 is an internal addition of a samarium ketyl to a triple bond.51 The resulting cyclic intermediate (7) either can react with another molecule of samarium(II) iodide or, since 7 is a highly reactive radical, abstract a hydrogen atom from the solvent (THF). Either reaction can be part of a two-step sequence leading to the final product (Scheme 5).
.png?revision=1&size=bestfit&width=430&height=109)
.png?revision=1&size=bestfit&width=425&height=177)
2. Internal Addition to a Carbon–Oxygen Double Bond (Pinacol Formation)
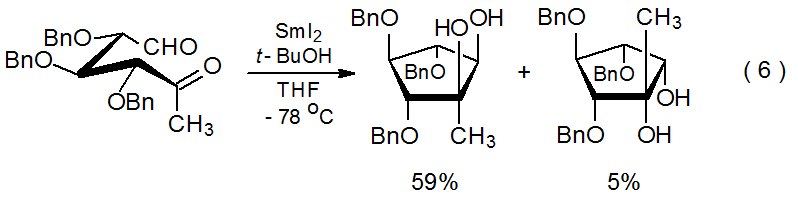
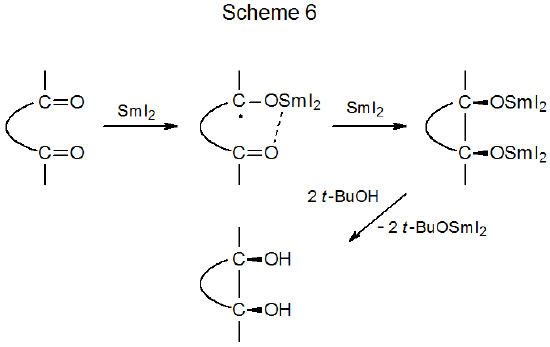
Reaction of samarium(II) iodide with a compound that has 1,4-,63 1,5‑,64–68 or 1,6‑69–77 related aldehydo or keto groups produces a samarium ketyl that then forms a cyclic pinacol. A typical example of such a reaction is shown in eq 6,64 and a general mechanism for pinacol formation is proposed in Scheme 6.78 Based on this proposal, one would expect that the two hydroxyl groups in a pinacol should be found on the same side of the newly formed ring system because during reaction the oxygen atoms in these two groups interact simultaneously with a single samarium ion. Further, one also would anticipate that reaction should place the hydroxyl groups stereoselectively on the less-hindered face of the new ring. Both of these expectations are realized not only in the reaction shown in eq 6 but in other, similar reactions, where the major products always are cis diols formed by minimizing steric interactions during ring construction.63,65–77
.png?revision=1&size=bestfit&width=400&height=100)
3. Internal Addition to a Carbon–Nitrogen Double Bond
Reaction analogous to pinacol formation occurs when one of the carbonyl groups in a reactant molecule is replaced by a group with a C–N double bond (eq 779).79–83 A significant stereochemical difference between this type of reaction and pinacol formation is that the hydroxyl and substituted amino groups produced during cyclization are on opposite faces of the newly formed ring. This result indicates that complexation between the carbonyl groups and the samarium ion during pinacol formation has no analogous interaction in reactions of keto-oximes.
.png?revision=1&size=bestfit&width=365&height=146)
Sometimes the cyclization of a keto-oxime produces an amine rather than a substituted amine (eq 7).79,84 This occurs when samarium(II) iodide, in excess of that needed for cyclization, transfers an electron to the N–O bond in the cyclic product leading to replacement of the amine substituent with a hydrogen atom. This reaction is accelerated by addition of water to the reaction mixture.
4. Ring-Contraction Reactions
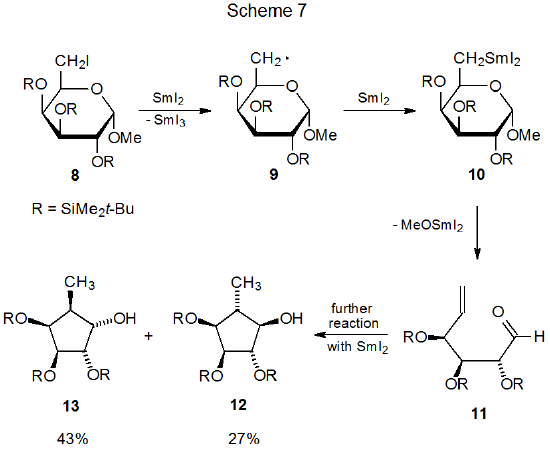
Scheme 7 describes a reaction in which a samarium ketyl is involved in ring contraction. This process begins with electron transfer from SmI2 to the carbohydrate iodide 8 to generate the radical 9.85 Reaction of 9 with a second molecule of SmI2 produces the organosamarium compound 10. Elimination of the elements of MeOSmI2 from 10 causes the pyranoid ring to open to give the unsaturated aldehyde 11, which reacts with SmI2 to form a samarium ketyl that then cyclizes to give the substituted cyclopentanes 12 and 13. Similar ring contractions occur when 6-aldehydo hexopyranosyl derivatives react with SmI2.86,87