V. Cyclization Reactions
- Page ID
- 24628
A. Substrates for Radical Cyclization
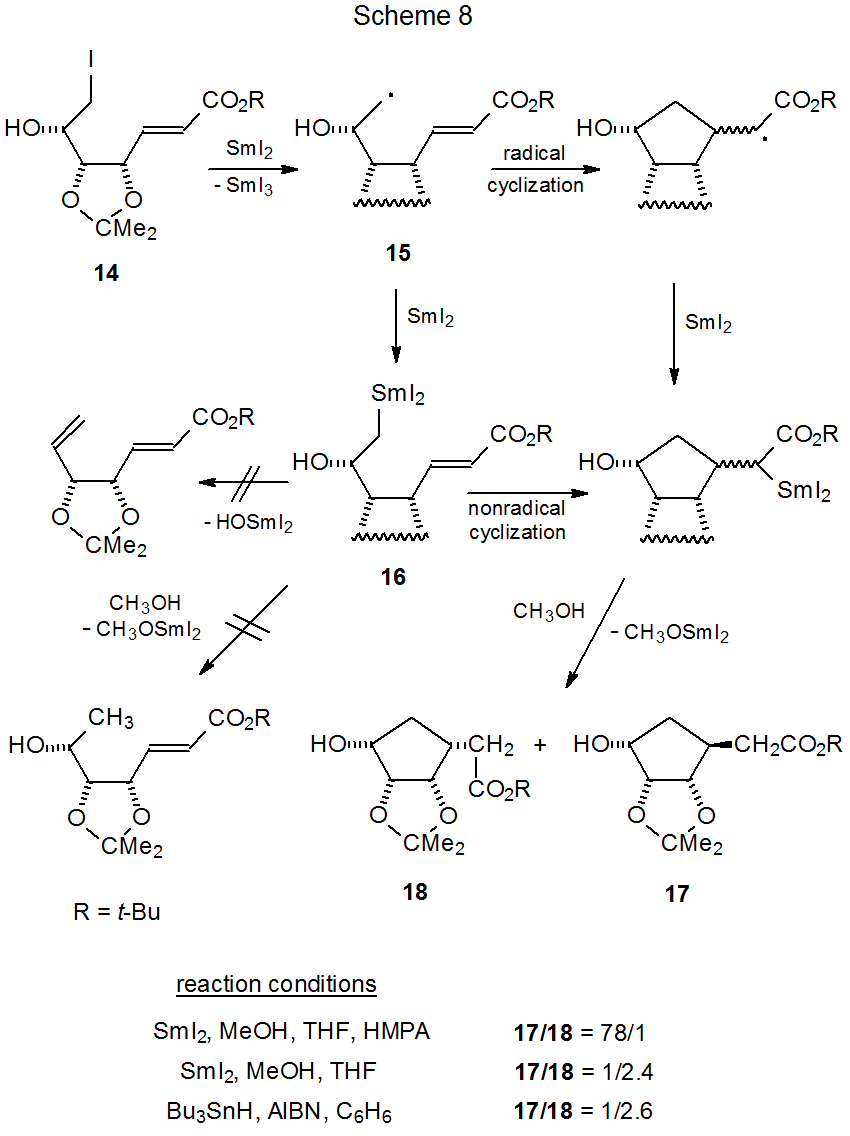
Radicals capable of cyclization can be generated from reaction of SmI2 with unsaturated carbohydrate sulfones13,27,88–91 or halides.14–18,92,93 Internal addition is possible to either a C–C14–18 (Scheme 8)14 or C–N92,93 (eq 8)92 double bond. Glycosyl phenyl sulfones are often the starting materials of choice for forming pyranos-1-yl radicals because not only are such sulfones more stable32 than the corresponding iodides and bromides, but they also produce radicals readily upon reaction with SmI2 in the presence of HMPA (Scheme 3). HMPA is critical to phenyl sulfone reactivity because in the absence of this cosolvent these sulfones are unreactive.13,91
.png?revision=1&size=bestfit&width=355&height=91)
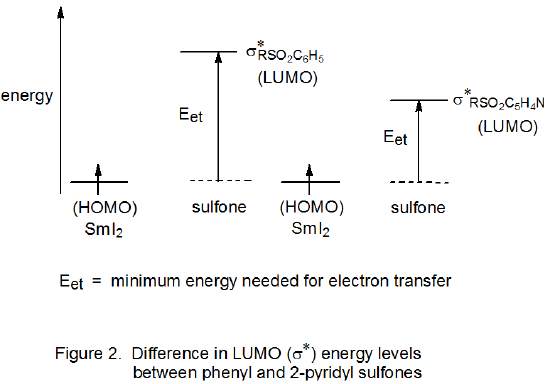
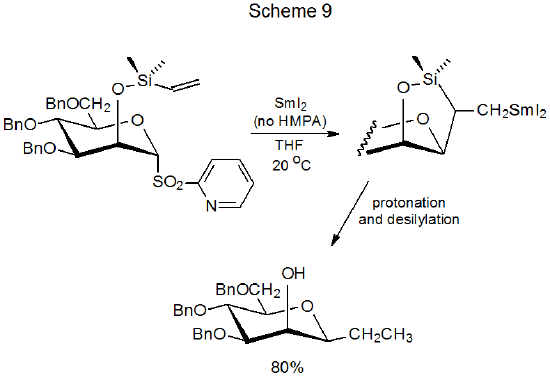
In contrast to phenyl sulfones, HMPA is not required for reaction of 2‑pyridyl sulfones. This contrasting behavior is attributed to the effect of the 2-pyridyl group on sulfone MO energy levels. Because the LUMO energy of a 2‑pyridyl sulfone is lower than that of a phenyl sulfone, transfer of an electron to the 2‑pyridyl derivative occurs more easily (Figure 2); as a result, reaction can take place without HMPA being present (Scheme 9).13,91
B. Radical Cyclization Versus Cyclization of an Organosamarium Compound
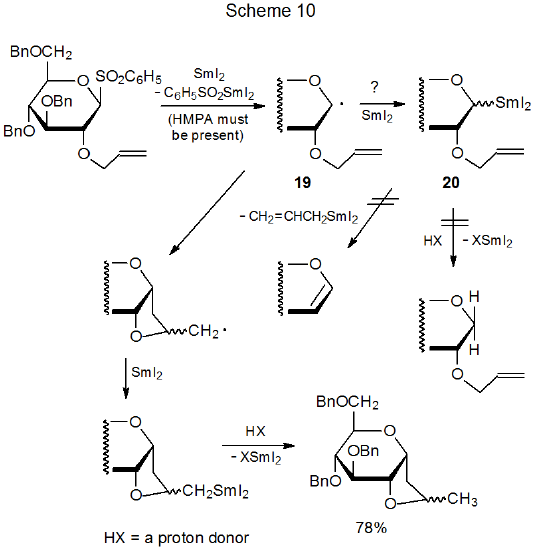
When single-electron transfer takes place from SmI2 to a substrate molecule, it often is not clear whether the reactive species is a radical, an organosamarium compound, or even an anion.94 In the reaction shown in Scheme 10, for example, radical cyclization and organosamarium compound formation are both possible from the radical 19.27 Since neither protonation nor β elimination, characteristic reactions of an organosamarium intermediate, is observed, the indication is that the radical 19 undergoes cyclization before formation of the organosamarium compound 20 can take place.
The reaction pictured in Scheme 814 is similar to the one shown in Scheme 10 in that ring formation occurs without the simple reduction or β elimination that characterize organosamarium intermediates. In this reaction (Scheme 8) stereoselectivity is highly dependent on the reaction conditions. For the AIBN initiated reaction of 14 with tri-n-butyltin hydride, there is little doubt that radical cyclization is taking place. The similarity in product ratios between this reaction and that caused by SmI2 (in the absence of HMPA) supports the idea that both reactions involve radical cyclization.
There is a dramatic change in stereoselectivity when HMPA is added to the reaction shown in Scheme 8.14 This change has been attributed to an HMPA-complexed samarium ion becoming associated with the carbonyl group in 15. The size of this group is believed to be sufficient to create severe steric interaction with the isopropylidene group, an interaction that forces these two groups to opposite faces of the newly formed ring.14 It is also possible, however, that the large change in stereoselectivity signals a new reaction mechanism. Cyclization may occur from the organosamarium intermediate 16. For this to happen, however, it would require 16 to be formed faster than internal radical addition to an activated double bond. It also would require cyclization of the organosamarium compound to be faster than protonation or β elimination from 16. The available information does not provide a definitive, mechanistic choice for this reaction, that is, the radical cyclization shown in Scheme 8 when HMPA is present.14

