VI. Radical Addition and Hydrogen-Atom Abstraction
- Page ID
- 24629
The reaction shown in Scheme 11 describes the formation of the C‑glycoside 25 by addition of the oxygen-stabilized radical 21 or the organosamarium compound 22 (or both) to a molecule of acetone.95 There is evidence for participation of both of these intermediates at some stage in this reaction. Conducting the reaction in the presence of t-BuSH quenches the addition process and dramatically increases the yield of the reduction product 23. Such a change would be expected from hydrogen-atom abstraction by the radical 21. In the absence of t-BuSH, formation of 23 and the elimination product 24 provide evidence for the organosamarium compound 22 also being present in the reaction mixture. Since conducting the reaction in the presence of D2O decreases the yield of the C-glycoside 25 in favor of the reduction and elimination products 23 and 24, respectively, the organosamarium compound 22 appears to be a likely intermediate in the addition process, but since a large excess of D2O only modestly reduces the yield of 25, radical addition remains a possible (perhaps major) pathway to C-glycoside formation.
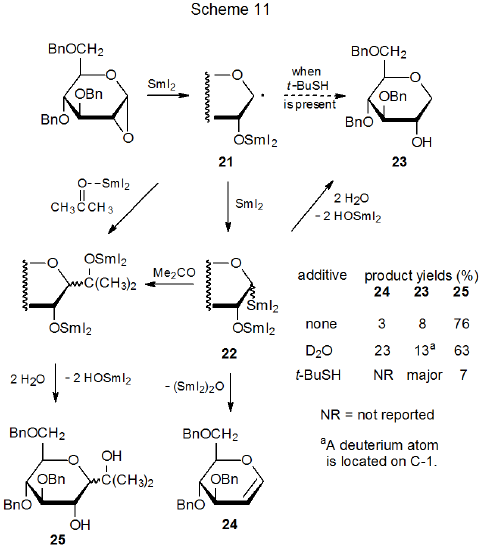
The radical-addition pathway shown in Scheme 11 involves the nucleophilic, carbon-centered radical 21 adding to the carbonyl carbon atom in acetone. The carbonyl carbon atom is rendered quite electron deficient by complexation of acetone with SmI2. This combination of a reactive radical adding to a double bond with a decidedly electron-deficient atom is found in other reactions promoted by SmI2.96,97

