II. Electron Transfer from a Redox Couple
- Page ID
- 24662
A. Direct Electron Donation
Electron donation from the metal in a redox couple to a halogenated carbohydrate can occur either directly or indirectly. With direct reaction the role of the metal ion is primarily to prepare the surface of the metal for interaction with the halogenated compound. This is believed to be the purpose of the copper ion in a zinc–copper couple, a reagent that has been described as an active form of zinc metal.1 Equation 1 pictures an addition reaction in which the adding radical is generated by reaction of a deoxyiodo sugar with a zinc–copper couple (Zn and CuI) suspended in an ethanol-water solution.2
.png?revision=1&size=bestfit&width=410&height=163)
B. Indirect Electron Donation
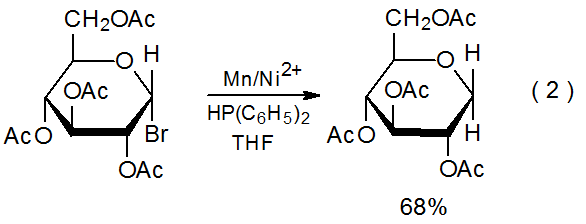
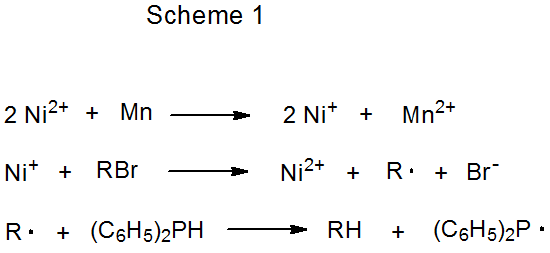
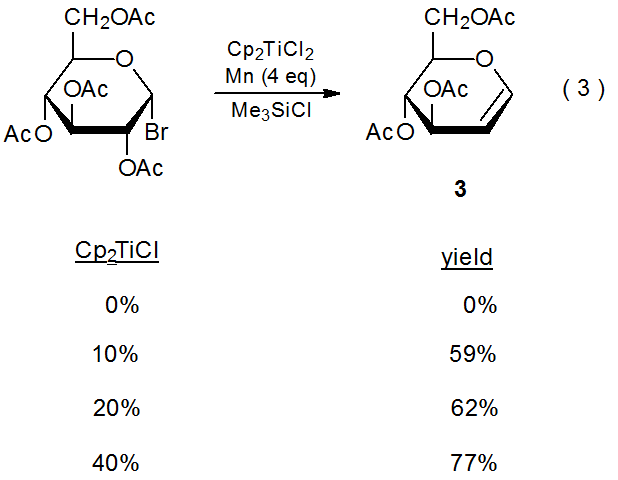
Indirect electron donation from the metal in a redox couple occurs when the metal ion is actively involved in the transfer process.3–5 An example of this type of participation is shown in eq 2, where Ni(I) is oxidized to Ni(II) during reaction with a halogenated carbohydrate, and Ni(II) then is reduced to Ni(I) by the manganese metal.3 Since the electrons being transferred to the carbohydrate are coming indirectly from manganese, the nickel ion performs a delivery role in the reaction and the manganese is the stoichiometric reactant (Scheme 1). In reactions of this type the metal ion need be present in only catalytic amounts; for example, in the glycal formation pictured in eq 3, the complex containing the titanium ion is added in as little as 10 mol%.4
.png?revision=1&size=bestfit&width=295&height=113)
.png?revision=1&size=bestfit&width=310&height=241)

