IV. Compounds with Phosphorous–Hydrogen Bonds
- Page ID
- 24669
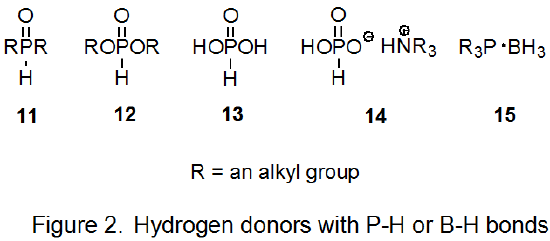
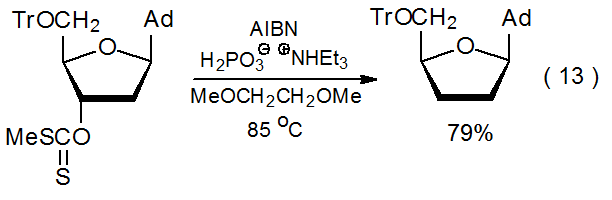
The search for less problematic hydrogen-atom transfers for use in the Barton-McCombie reaction has led to compounds with phosphorus–hydrogen bonds. These include dialkylphosphine oxides (11), alkyl phosphites (12), hypophosphorous acid (13), and salts of hypophosphorous acid (14) (Figure 2). All of these compounds can function as inexpensive, nontoxic hydrogen-atom transfers that form the chain-carrying radicals needed for reaction and do not produce byproducts difficult to remove.3,10,57 An example of a reaction in which hydrogen donation is from a P–H bond is shown in eq 13.58
.png?revision=1&size=bestfit&width=310&height=101)
Alkyl phosphites (12) are excellent hydrogen-atom transfers, but reactions involving these compounds have the disadvantage of not being able to be initiated by 2,2'-azobis(isobutyronitrile); benzoyl peroxide usually is the initiator.3,10 Reactions in which the hydrogen-atom transfer is a dialkylphosphine oxide (11), hypophosphorous acid (13), or a salt of hypophosphorous acid (14) can be initiated by AIBN.9,57 Because it is difficult to completely remove water from hypophosphorous acid and its salts, these donors are less attractive choices when moisture sensitive compounds are reacting.9

