VI. Compounds with Carbon–Hydrogen Bonds
- Page ID
- 24671
A. 2-Propanol
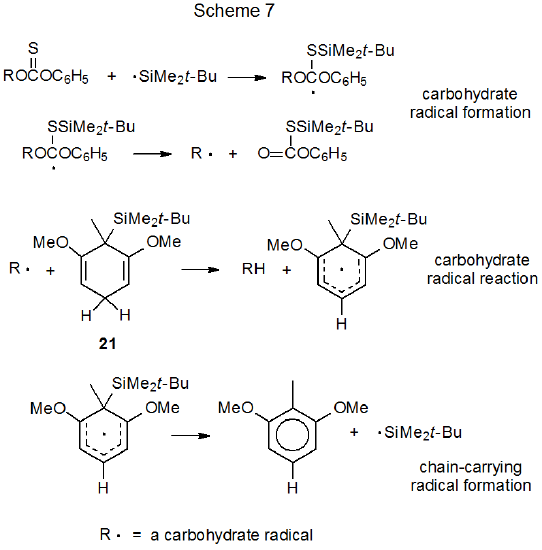
Few compounds in which a carbon–hydrogen bond must serve as the hydrogen-atom source are reactive enough to function as hydrogen-atom transfers in radical reactions of carbohydrates. The reason for this is that when less reactive donors are used, other reactions become competitive. Even compounds with quite reactive C–H bonds are poor hydrogen-atom transfers when compared to tri-n-butyltin hydride or tris(trimethylsilyl)silane. One compound that does have the necessary reactivity, but just barely, is 2‑propanol. When reaction of the xanthate 16 is conducted with 2‑propanol as the solvent, hydrogen-atom abstraction is in spirited competition with xanthate-dithiocarbonate rearrangement (eq 15).59 This competition exists because hydrogen-atom abstraction by the carbohydrate radical R· is slow enough that addition of R· to another molecule of the xanthate 16 has a comparable rate (Scheme 5). The adduct radical formed by this addition fragments to give the dithiocarbonate 18 and a carbohydrate radical (R·).
.png?revision=1&size=bestfit&width=420&height=145)
B. Cyclohexane
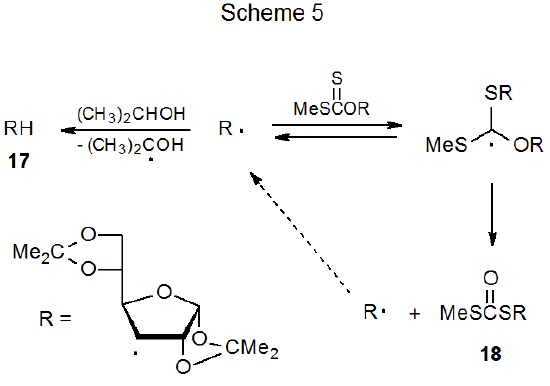
The xanthate 19 reacts to form the corresponding deoxy sugar in 85% yield (eq 16).60 In this reaction cyclohexane functions as the hydrogen-atom transfer. Since cyclohexane is not a noticeably better hydrogen-atom transfer than 2-propanol, it is initially surprising that no dithiocarbonate is formed from 19 even though (as described in the previous section) dithiocarbonate formation is significant in reaction of the xanthate 16 (eq 15). The structural difference between the starting materials (16 and 19) in these two reactions accounts for their difference in reactivity. Unlike 16, the xanthate 19 has a sulfur atom directly attached to the carbohydrate portion of the molecule. This means that when the carbohydrate radical R· adds to 19, the options available to the adduct radical 20 are either regenerating the starting materials or expelling an unstabilized, primary radical (Scheme 6). Not surprisingly, no dithiocarbonate from primary radical expulsion is observed; therefore, the only operative pathway for the radical 20 is reforming of R· and the xanthate 19. Each regeneration of R· creates a new opportunity for it to abstract a hydrogen atom. With these multiple opportunities even a marginally effective hydrogen-atom transfer eventually is able to react with R· to produce the hydrogen-abstraction product RH.
.png?revision=1&size=bestfit&width=350&height=110)
Even though the yield is good, the reaction shown in eq 16 is not an attractive option for deoxy sugar synthesis because it requires reaction of the carbohydrate to replace a C–O bond with a C–S bond before conducting the Barton-McCombie reaction. The additional steps necessary for this conversion add to the effort required for deoxygenation.
C. Silylated Cyclohexadienes
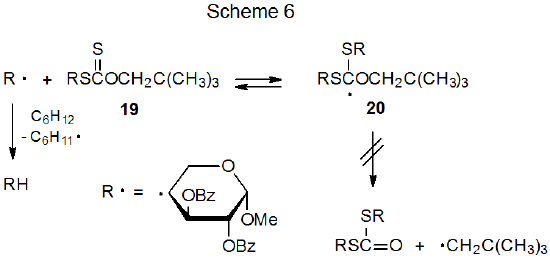
Silylated cyclohexadienes, such as 21, are effective hydrogen-atom transfers in Barton-McCombie reactions (eq 17).61 Compound 21 has the advantage of being a solid material that can be easily stored and handled. Although this compound (21) is an order of magnitude less reactive than (Me3Si)3SiH (3), it is able to support chain reactions. The propagation steps in a proposed mechanism for replacement of an O-phenoxythiocarbonyl group with a hydrogen atom supplied by 21 are given in Scheme 7.
.png?revision=1&size=bestfit&width=430&height=218)