Diastereomers
- Page ID
- 796
Diastereomers are stereoisomers that are not related as object and mirror image and are not enantiomers. Unlike enatiomers which are mirror images of each other and non-sumperimposable, diastereomers are not mirror images of each other and non-superimposable. Diastereomers can have different physical properties and reactivity. They have different melting points and boiling points and different densities. They have two or more stereocenters.
Introduction
It is easy to mistake between diasteromers and enantiomers. For example, we have four steroisomers of 3-bromo-2-butanol. The four possible combination are SS, RR, SR and RS (Figure 1). One of the molecule is the enantiomer of its mirror image molecule and diasteromer of each of the other two molecule (SS is enantiomer of RR and diasteromer of RS and SR). SS's mirror image is RR and they are not superimposable, so they are enantiomers. RS and SR are not mirror image of SS and are not superimposable to each other, so they are diasteromers.
Figure 1
Diastereomers vs. Enantiomers vs. Meso Compounds
Tartaric acid, C4H6O6, is an organic compound that can be found in grape, bananas, and in wine. The structures of tartaric acid itself is really interesting. Naturally, it is in the form of (R,R) stereocenters. Artificially, it can be in the meso form (R,S), which is achiral. R,R tartaric acid is enantiomer to is mirror image which is S,S tartaric acid and diasteromers to meso-tartaric acid (figure 2).
(R,R) and (S,S) tartaric acid have similar physical properties and reactivity. However, meso-tartaric acid have different physical properties and reactivity. For example, melting point of (R,R) & (S,S) tartaric is about 170 degree Celsius, and melting point of meso-tartaric acid is about 145 degree Celsius.
Figure 2
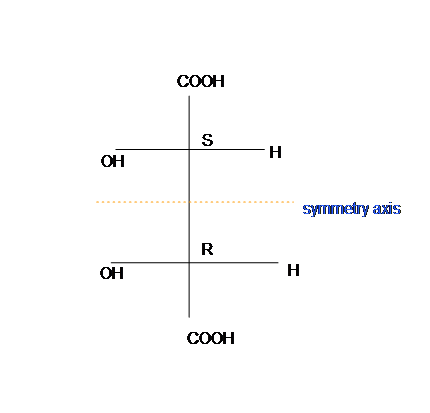
To identify meso, meso compound is superimposed on its mirror image, and has an internal plane that is symmetry (figure 3). Meso-tartaric acid is achiral and optically unactive.
Problems
Identify which of the following pair is enantiomers, diastereomers or meso compounds.
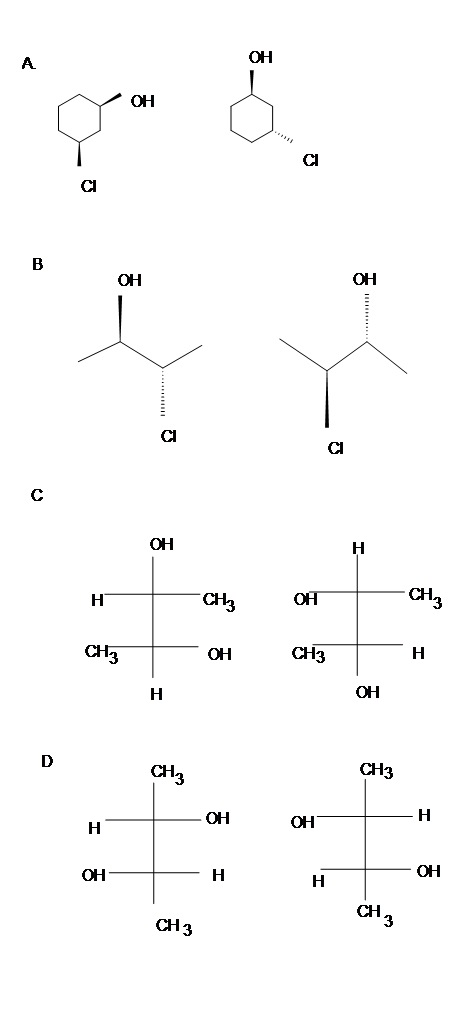
Answer
- Diasteromers
- Identical
- Meso
- Enantiomers


.png?revision=1&size=bestfit&width=720&height=519)
.bmp?revision=1&size=bestfit&width=720&height=567)