Polymerization of Conjugated Dienes
- Page ID
- 813
Conjugated dienes (alkenes with two double bonds and a single bond in between) can be polymerized to form important compounds like rubber. This takes place, in different forms, both in nature and in the laboratory. Interactions between double bonds on multiple chains leads to cross-linkage which creates elasticity within the compound.
Polymerization of 1,3-Butadiene
For rubber compounds to be synthesized, 1,3-butadiene must be polymerized. Below is a simple illustration of how this compound is formed into a chain. The 1,4 polymerization is much more useful to polymerization reactions.

Above, the green structures represent the base units of the polymers that are synthesized and the red represents the bonds between these units which form these polymers. Whether the 1,3 product or the 1,4 product is formed depends on whether the reaction is thermally or kinetically controlled.
Synthetic Rubber
The most important synthetic rubber is Neoprene which is produced by the polymerization of 2-chloro-1,3-butadiene.
.png?revision=1&size=bestfit&width=350&height=133)
In this illustration, the dashed lines represent repetition of the same base units, so both the products and reactants are polymers. The reaction proceeds with a mechanism similar to the Friedel-Crafts mechanism. Cross-linkage between the chlorine atom of one chain and the double bond of another contributes to the overall elasticity of neoprene. This cross-linkage occurs as the chains lie next to each other at random angles, and the attractions between double bonds prevent them from sliding back and forth.
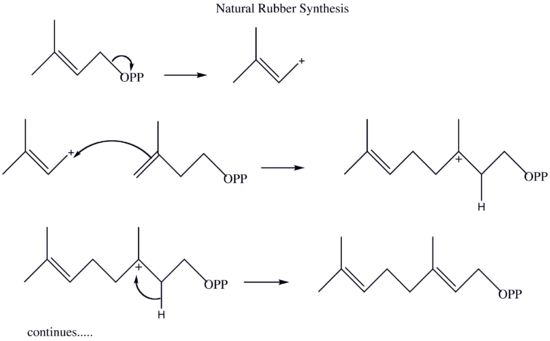
Natural Rubber
The synthesis of rubber in nature in somewhat similar the artificial synthesis of rubber except that it takes place within a plant. Instead of the 2-chloro-1,3-butadiene used in the synthesis of neoprene, natural rubber is synthesized from 2-methyl-1,3-butadiene. As an electrophile, the plant synthesizes the pyrophosphate 3-methyl-3-butenyl pyrophosphate is from phosphoric acid and 3-methyl-3-buten-1-ol. This pyrophosphate then catalyzes the reaction that leads to natural rubber.
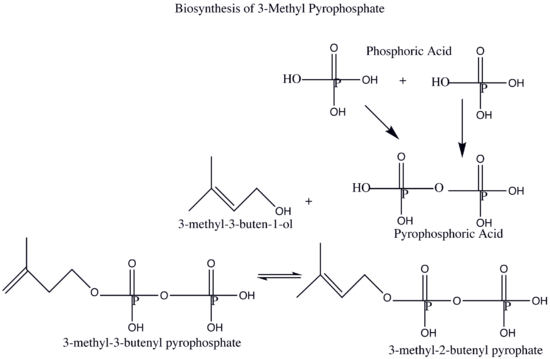
The 3-methyl-3-butenyl pyrophosphate (OPP) is then used in the polymerization of natural rubber as it pulls electrons off 2-methyl-1,3-butadiene (see questions section for this process.)
References
- Vollhardt, Peter, and Neil E. Schore. Organic Chemistry: Structure and Function. New York: W. H. Freeman & Company, 2007.
- Buehr, Walter. Rubber: Natural and Synthetic. Morrow, 1964.
Problem
Draw out the mechanism for the natural synthesis of rubber from 3-methyl-3-butenyl pyrophosphate and 2-methyl-1,3-butadiene. Show the movement of electrons with arrows.
Answer