Aldol Reaction
- Page ID
- 16585
Enolizable aldehydes and enolizable ketones, in the presence of an acid or base catalyst usually in aqueous medium at low temperature, undergo a reaction, giving an aldol as the major product. This reaction is known as aldol reaction. The base-catalyzed aldol reaction, in which the catalyst is usually the hydroxide ion, is more common. Careful control of reaction temperature is critical because high temperatures promote aldol condensation. The optimum temperature depends on the nature of the aldehyde or ketone. Typically, heating the reaction mixture results in aldol condensation.
eg:
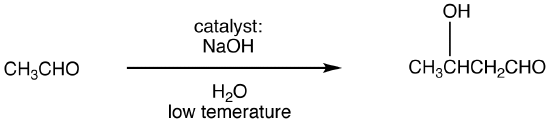
Mechanism:
Step 1: The hydroxide ion deprotanates the aldehyde reversibly.

Step 2: Enolate ion 1 adds to the unreacted aldehyde.

Step 3: Alkoxide ion 2 is protonated by water.
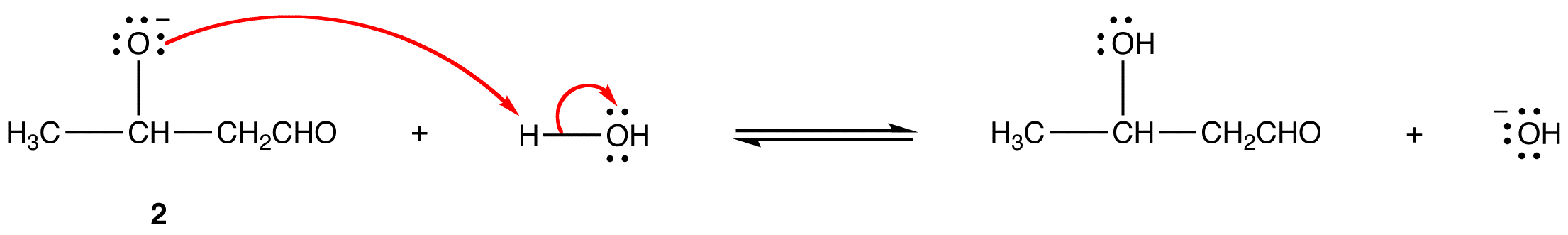
Since all three steps are reversible, the overall reaction is reversible and its equilibrium constant depends on the nature of the aldehyde or ketone. The reactions of aldehydes in which the α-carbon is a secondary carbon usually have large equilibrium constants; those of other aldehydes and ketones have small equilibrium constants. These observations suggest that the equilibrium constant of aldol reaction is sensitive to steric hindrance at the carbonyl carbon in the aldehyde or ketone.


