Radical Chain Reaction
- Page ID
- 42306
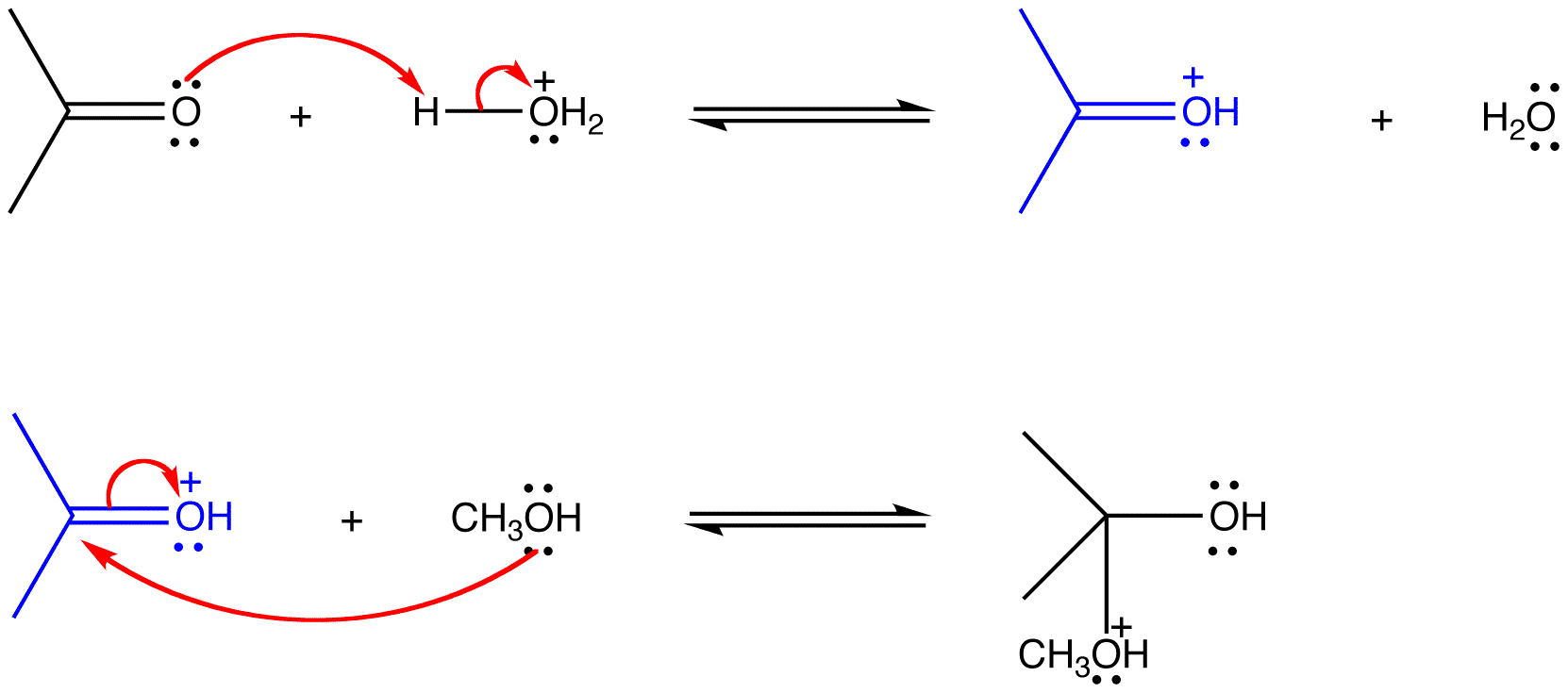
In a multi-step reaction involving ionic intermediates, a product or products of one step act as the reactants of a subsequent step.
eg: The following reactions are consecutive steps of the acid-catalyzed reaction of acetone with methanol.
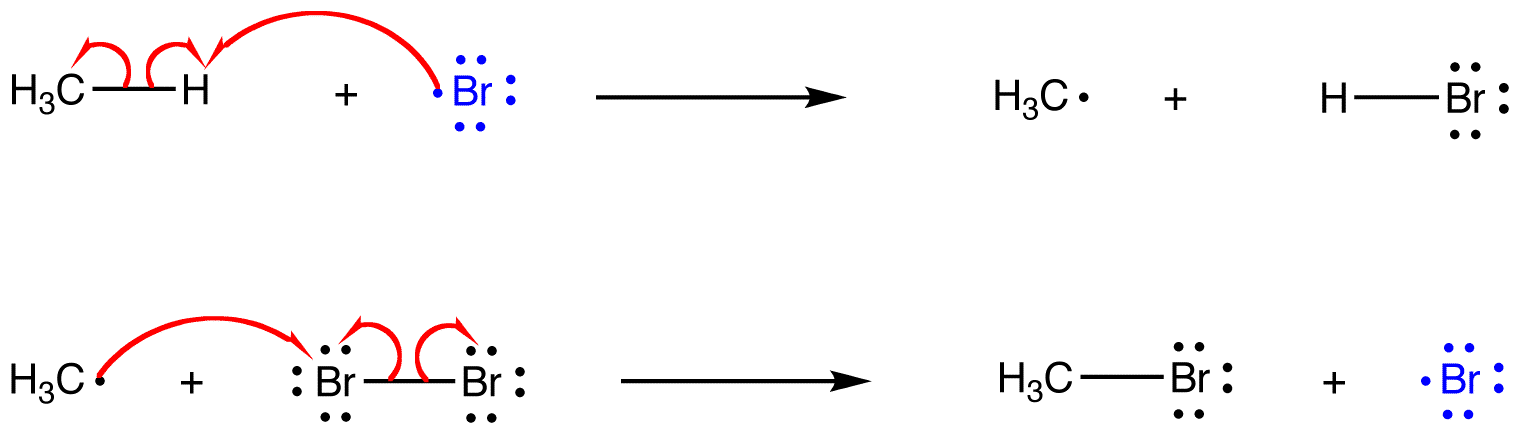
In a multi-step reaction involving radical intermediates, however, a radical reactant of one step is often the product of a subsequent step.
eg: The following reactions are consecutive steps of the heat-induced reaction of excess methane with bromine.
The regenerated bromine radical could react with a molecule of methane, starting another round of the two-reaction sequence. The process could continue until bromine, which is the limiting reagent, is completely consumed.
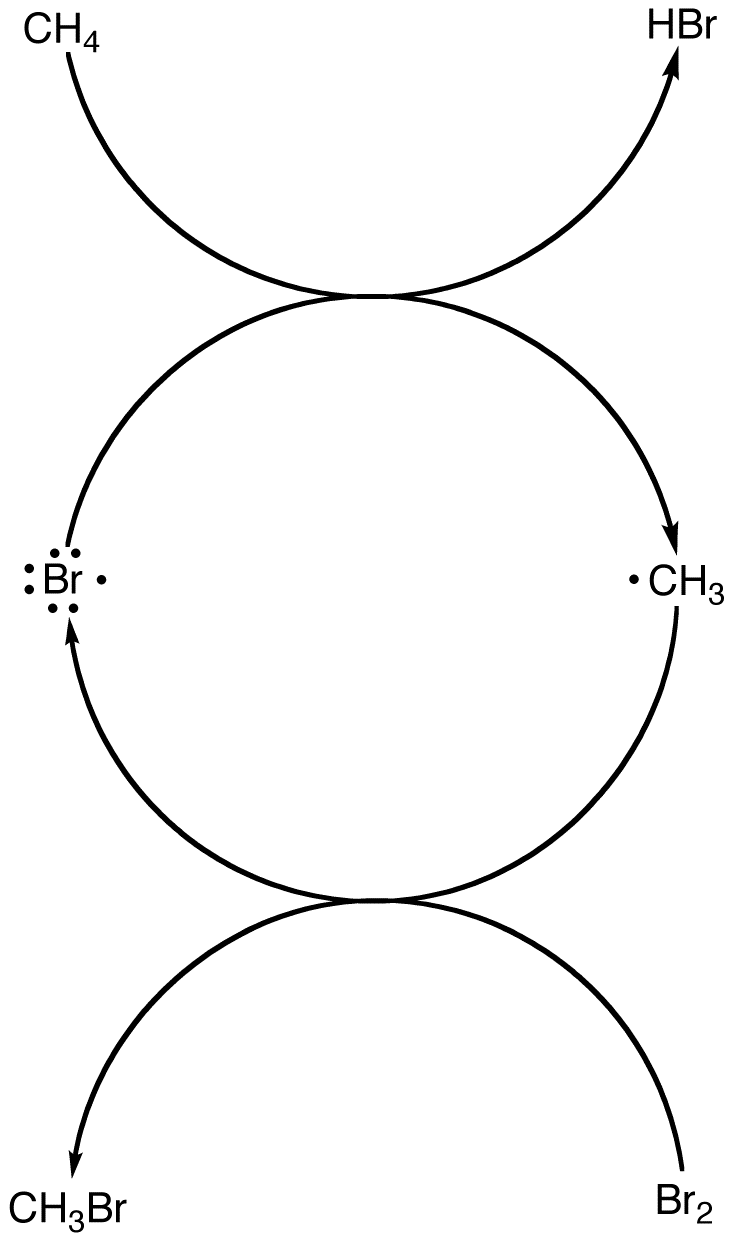
Due to recycling of the bromine radical, at least in theory, the reaction that generates the first bromine radical in the system has to occur only once.
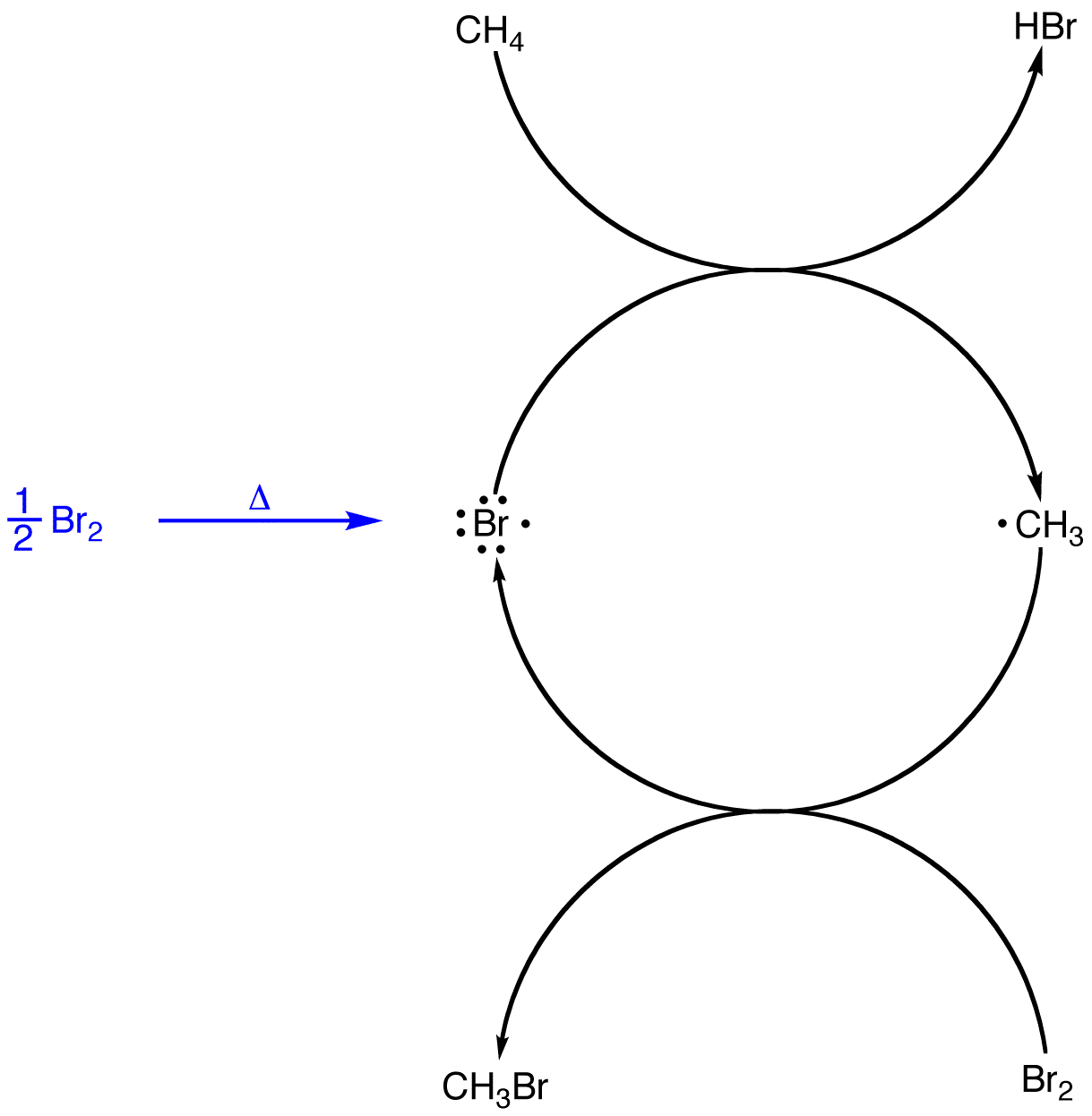
A multi-step reaction in which a radical is consumed in one step but regenerated in a subsequent step is called a chain reaction. The heat-induced reaction of excess methane with bromine is a chain reaction.
see initiation, propagation, termination


