Alkenes from Dehydration of Alcohols
- Page ID
- 905
One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo E1 or E2 mechanisms to lose water and form a double bond.
Introduction
The dehydration reaction of alcohols to generate alkene proceeds by heating the alcohols in the presence of a strong acid, such as sulfuric or phosphoric acid, at high temperatures.
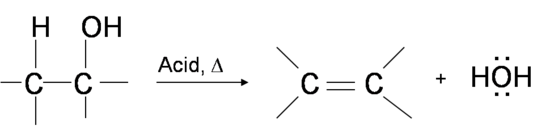
The required range of reaction temperature decreases with increasing substitution of the hydroxy-containing carbon:
- 1° alcohols: 170° - 180°C
- 2° alcohols: 100°– 140 °C
- 3° alcohols: 25°– 80°C
If the reaction is not sufficiently heated, the alcohols do not dehydrate to form alkenes, but react with one another to form ethers (e.g., the Williamson Ether Synthesis).
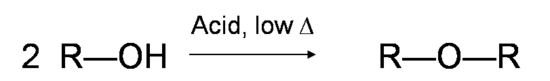
Alcohols are amphoteric; they can act both as acid or base. The lone pair of electrons on oxygen atom makes the –OH group weakly basic. Oxygen can donate two electrons to an electron-deficient proton. Thus, in the presence of a strong acid, R—OH acts as a base and protonates into the very acidic alkyloxonium ion +OH2 (The pKa value of a tertiary protonated alcohol can go as low as -3.8). This basic characteristic of alcohol is essential for its dehydration reaction with an acid to form alkenes.
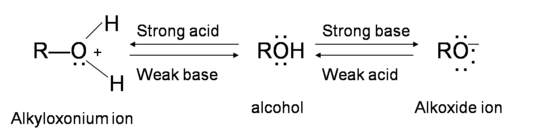
Mechanism for the Dehydration of Alcohol into Alkene
Different types of alcohols may dehydrate through a slightly different mechanism pathway. However, the general idea behind each dehydration reaction is that the –OH group in the alcohol donates two electrons to H+ from the acid reagent, forming an alkyloxonium ion. This ion acts as a very good leaving group which leaves to form a carbocation. The deprotonated acid (the nucleophile) then attacks the hydrogen adjacent to the carbocation and form a double bond.
Primary alcohols undergo bimolecular elimination (E2 mechanism) while secondary and tertiary alcohols undergo unimolecular elimination (E1 mechanism). The relative reactivity of alcohols in dehydration reaction is ranked as the following
Methanol < primary < secondary < tertiary
Primary alcohol dehydrates through the E2 mechanism
Oxygen donates two electrons to a proton from sulfuric acid H2SO4, forming an alkyloxonium ion. Then the nucleophile HSO4– back-side attacks one adjacent hydrogen and the alkyloxonium ion leaves in a concerted process, making a double bond.

Secondary and tertiary alcohols dehydrate through the E1 mechanism
Similarly to the reaction above, secondary and tertiary –OH protonate to form alkyloxonium ions. However, in this case the ion leaves first and forms a carbocation as the reaction intermediate. The water molecule (which is a stronger base than the HSO4- ion) then abstracts a proton from an adjacent carbon, forming a double bond. Notice in the mechanism below that the aleke formed depends on which proton is abstracted: the red arrows show formation of the more substituted 2-butene, while the blue arrows show formation of the less substituted 1-butene. Recall the general rule that more substituted alkenes are more stable than less substituted alkenes, and trans alkenes are more stable than cis alkenes. Therefore, the trans diastereomer of the 2-butene product is most abundant.
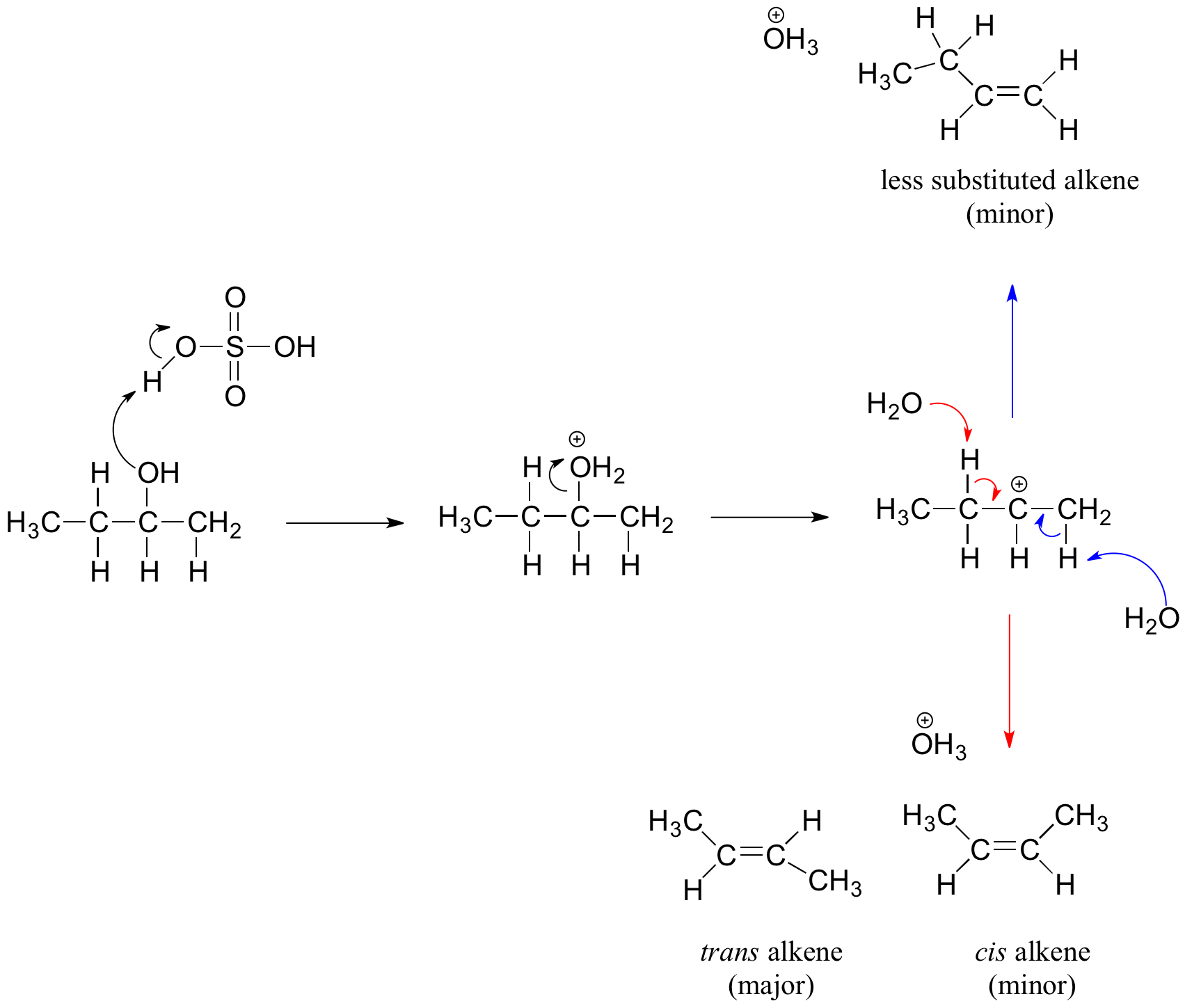
Dehydration reaction of secondary alcohol: The dehydration mechanism for a tertiary alcohol is analogous to that shown above for a secondary alcohol.
When more than one alkene product are possible, the favored product is usually the thermodynamically most stable alkene. More-substituted alkenes are favored over less-substituted ones; and trans-substituted alkenes are preferred compared to cis-substituted ones.
- Since the C=C bond is not free to rotate, cis-substituted alkenes are less stable than trans-subsituted alkenes because of steric hindrance (spatial interfererence) between two bulky substituents on the same side of the double bond (as seen in the cis product in the above figure). Trans-substituted alkenes reduce this effect of spatial interference by separating the two bulky substituents on each side of the double bond (for further explanation on the rigidity of C=C bond, see Structure and Bonding in Ethene- The pi Bond).
- Heats of hydrogenation of differently-substituted alkene isomers are lowest for more-substituted alkenes, suggesting that they are more stable than less-substituted alkenes and thus are the major products in an elimination reaction. This is partly because in more --substituted alkenes, the p orbitals of the pi bond are stabilized by neighboring alkyl substituents, a phenomenon similar to hyperconjugation.
Hydride and Alkyl Shifts
Since the dehydration reaction of alcohol has a carbocation intermediate, hydride or alkyl shifts can occur which relocates the carbocation to a more stable position. The dehydrated products therefore are a mixture of alkenes, with and without carbocation rearrangement. Tertiary cation is more stable than secondary cation, which in turn is more stable than primary cation due to a phenomenon known as hyperconjugation, where the interaction between the filled orbitals of neighboring carbons and the singly occupied p orbital in the carbocation stabilizes the positive charge in carbocation.
- In hydride shifts, a secondary or tertiary hydrogen from a carbon next to the original carbocation takes both of its electrons to the cation site, swapping place with the carbocation and renders it a more stable secondary or tertiary cation.
Similarly, when there is no hydride available for hydride shifting, an alkyl group can take its bonding electrons and swap place with an adjacent cation, a process known as alkyl shift.
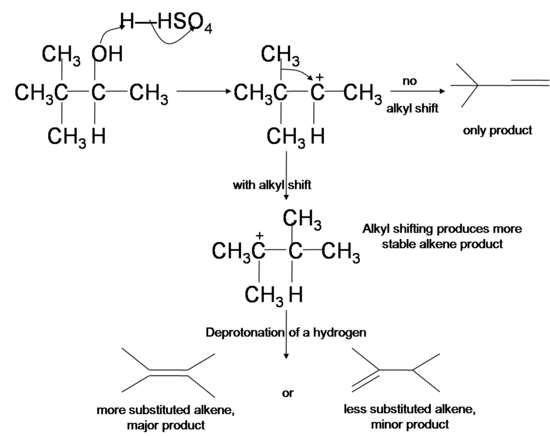
Practice Problems
Test your understanding by predicting what product(s) will be formed in each of the following reactions:
1.
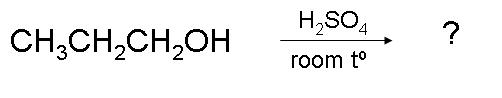
2.
Solutions
1. Did you notice the reaction temperature? It is only 25°, which is much lower than the required temperature of 170°C for dehydration of primary alcohol. This reaction will not produce any alkene but will form ether.
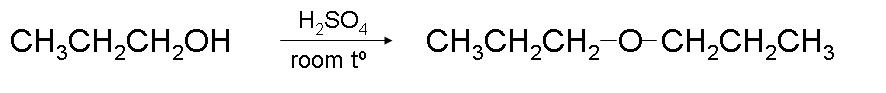
2. . Notice that the reactant is a secondary -OH group, which will form a relatively unstable secondary carbocation in the intermediate. Thus hydride shift from an adjacent hydrogen will occur to make the carbocation tertiary, which is much more stable. The products are a mixture of alkenes that are formed with or without carbocation rearrangement (A number of products are formed faster than hydride shift can occur).
References
- Vollhart, K. Peter C. and Neil Schore. Organic Chemistry, Structure and Function. 5th Ed. W. H. Freeman and Company, 2007
- McMurry, John. Fundamentals of Organic Chemistry. 3rd Ed. Cornell University. Pacific Grove, CA: Brooks/ Cole Publishing Company, 1994.
Contributors
- Thuy Hoang

