15.9: Outside the box - 1,3-elimination and rearrangement in squalene synthase
- Page ID
- 1002
In this section, we will briefly examine the reaction catalyzed by an enzyme called squalene synthase, an important enzymatic transformation that involves some very interesting and unusual electrophilic additions, rearrangements, and reactive intermediates. This particular enzyme is also of interest because it represents a potential new target for cholesterol-lowering drugs.
Cholesterol, as we discussed earlier in this chapter, is derived from a 30-carbon isoprenoid molecule called squalene. Squalene, in turn, is derived from the condensation of two molecules of farnesyl diphosphate (FPP), a 15-carbon isoprenoid. You may recall (section 15.3B) that FPP is the product of the C4 to C1, or 'head to tail' electrophilic condensation of isoprenoid chains:
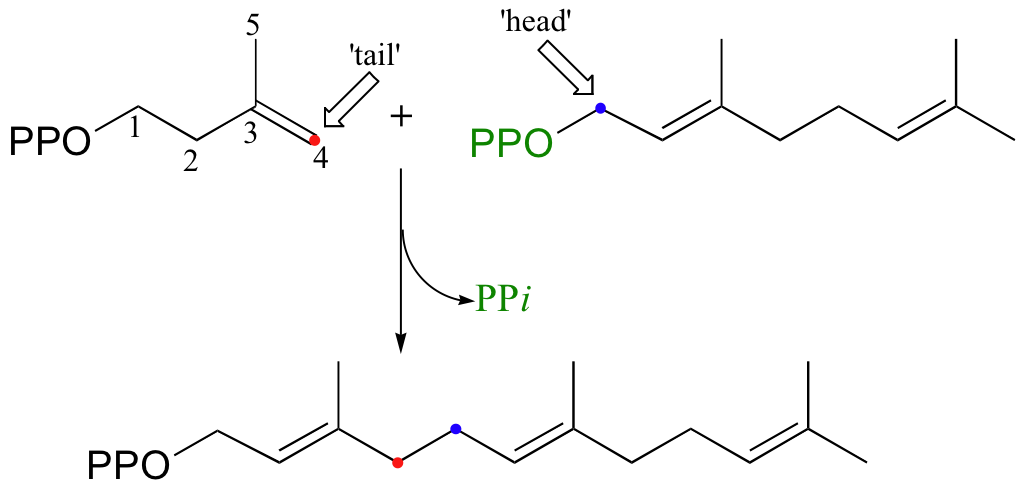
The condensation of two molecules of FPP to form squalene, however, is something different: this is a 'head to head' condensation, where C1 of the first molecule forms a bond to C1 of the second. The chemistry involved is quite a bit more complicated.
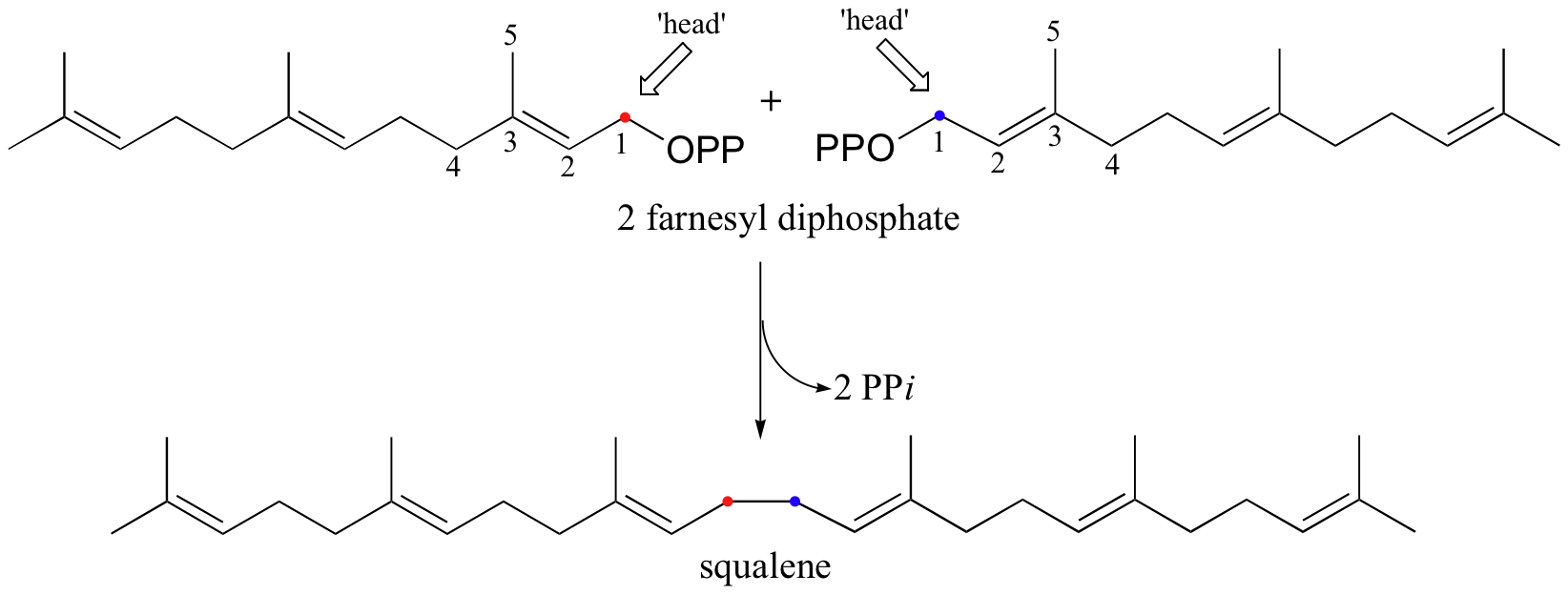
The first two steps are familiar: first, the pyrophosphate on one FPP molecule leaves (step 1), resulting in an allylic carbocation that is attacked by the C2-C3 π bond of the second molecule (step 2).
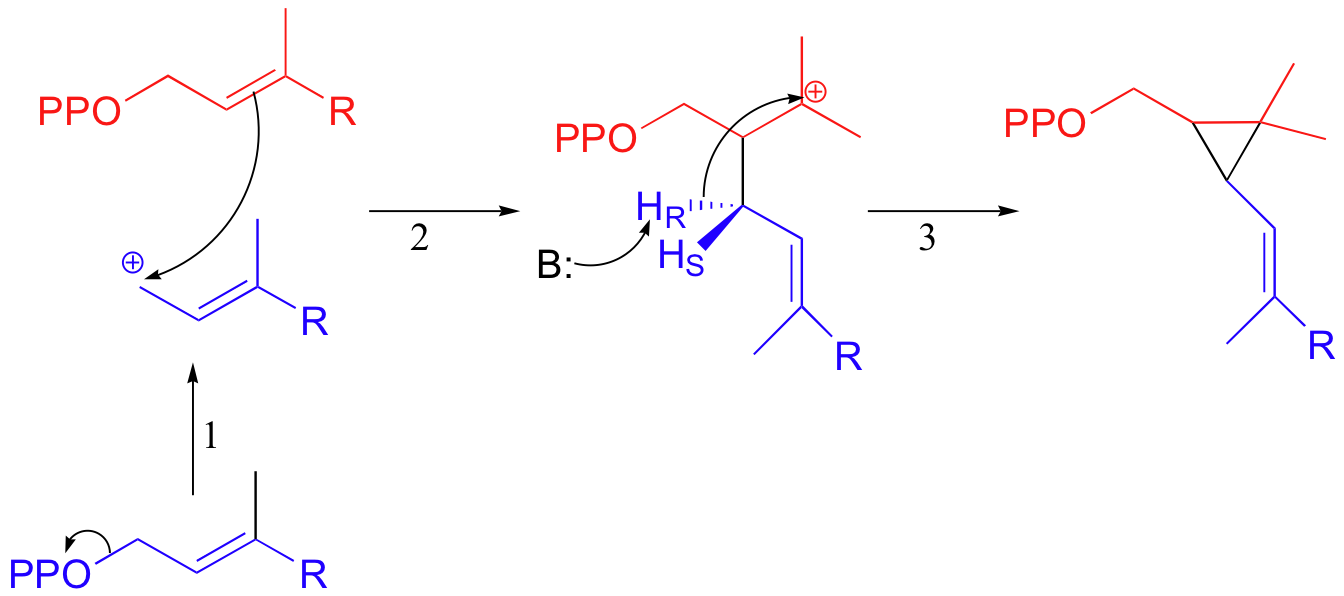
This results in a new carbon-carbon bond between the two FPP molecules, but with incorrect C1 to C2 connectivity (remember, the overall reaction is a C1 to C1 condensation). In step 3, a proton is abstracted and the electrons from the broken C-H bond bridge across a 2-carbon gap to form a cyclopropyl intermediate.
In the second stage of squalene synthesis, the second pyrophosphate group leaves, generating a cyclopropylcarbinyl cation (step 4). Because this is a primary carbocation, you probably are wondering about how stable it could be (and thus how likely an intermediate). As it turns out, such carbocations are remarkably stable, due to favorable interactions between the empty orbital and orbitals on the three-membered ring (the level of bonding theory needed to really understand this idea is beyond the scope of this text, but you may learn about it if you take a class in advanced organic chemistry). What occurs next is an alkyl shift leading to a tertiary carbocation (step 5).
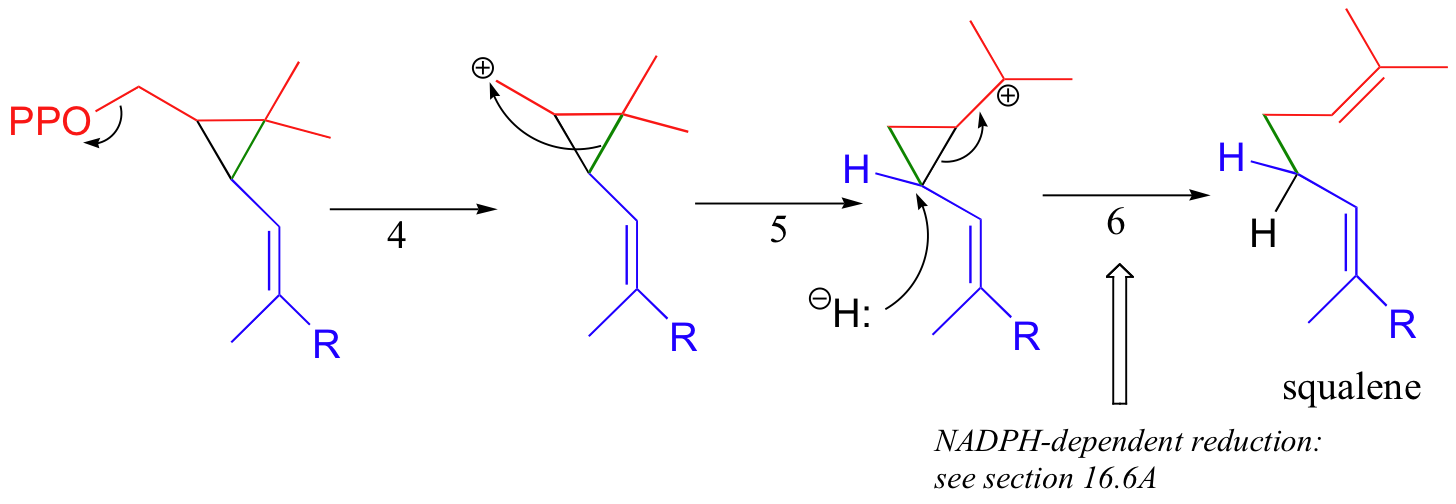
Discussion of the final step (step 6) will need to be put off until chapter 16 - this is a reduction with a hydride nucleophile derived from a coenzyme called NADPH.
Although this may seem like an extremely convoluted (and perhaps unlikely!) mechanism, there is much experimental evidence to back it up. In the next chapter (section 16.6A), we will come back to this reaction and discuss some of the evidence pointing to the existence of one of the proposed intermediates.


