15: Electrophilic reactions
- Page ID
- 992
Up to this point in our study of organic reaction mechanisms, we have seen two fundamental types of electron-rich organic species that can act as nucleophiles or bases. First, in chapters 8 through 12, we studied reactions in which heteroatoms such as oxygen, nitrogen, and sulfur acted as nucleophiles and bases. Next, in chapters 13 and 14, we saw how carbanions, stabilized in the form of enolates, enols, enamines, or coenzyme adducts, can act as nucleophiles or bases. In this chapter, we focus on a third possibility: reactions in which the electrons in a carbon-carbon pi bond react in a nucleophilic or basic fashion:
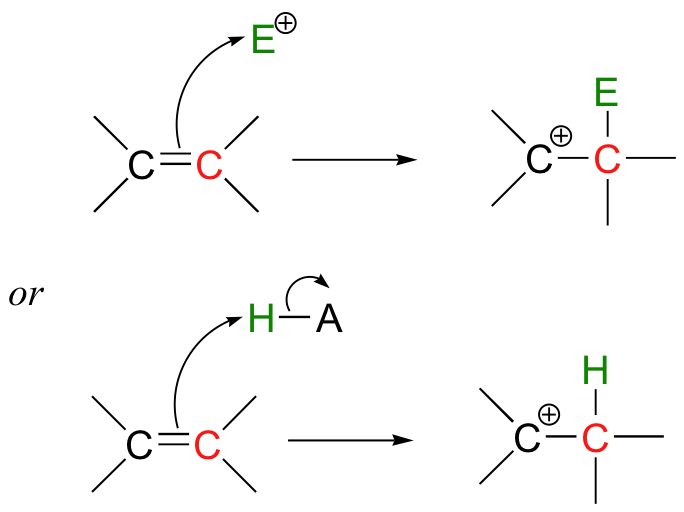
These reactions are often referred to as 'electrophilic' - which is actually a bit confusing. Every reaction we have studied so far has involved both an electrophile and a nucleophile, so what does it mean to refer to an 'electrophilic' reaction? It turns out that as organic terminology has evolved, reaction steps involving pi-bonded electrons acting as nucleophiles or bases are called 'electrophilic' steps. This is because pi bonds, compared to oxygens, nitrogens, sulfurs, and enolates, etc., are only weakly nucleophilic, so they require a powerful electrophilic partner. The emphasis, in other words, is on the electrophilic side of the equation, hence the name. But don't let the nomenclature confuse you - these reactions, like all other reactions in organic chemistry, involve the interaction between an electron-rich species (a nucleophile or base, in this chapter a pair of pi-bonded electrons), and a electron-poor species (an electrophile or a proton).
Additionally, we will see in this chapter an interesting new twist on organic reactivity, when we study how hydrogens and alkyl groups can 'shift', or 'rearrange' from carbocation intermediates.
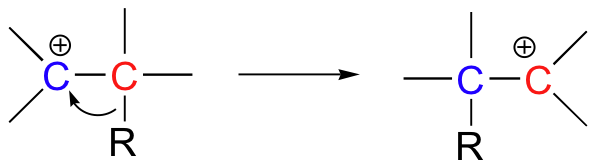
Because most of the reactions in this chapter involve one or more carbocation intermediates, the concept of carbocation stability (section 8.4) will be important - you may want to go back and review those ideas before tackling this chapter.


