17.2: Radical chain reactions
- Page ID
- 1021
17.2A: The three phases of radical chain reactions
Because of their high reactivity, free radicals have the potential to be both extremely powerful chemical tools and extremely harmful contaminants. Much of the power of free radical species stems from the natural tendency of radical processes to occur in a chain reaction fashion. Radical chain reactions have three distinct phases: initiation, propagation, and termination.
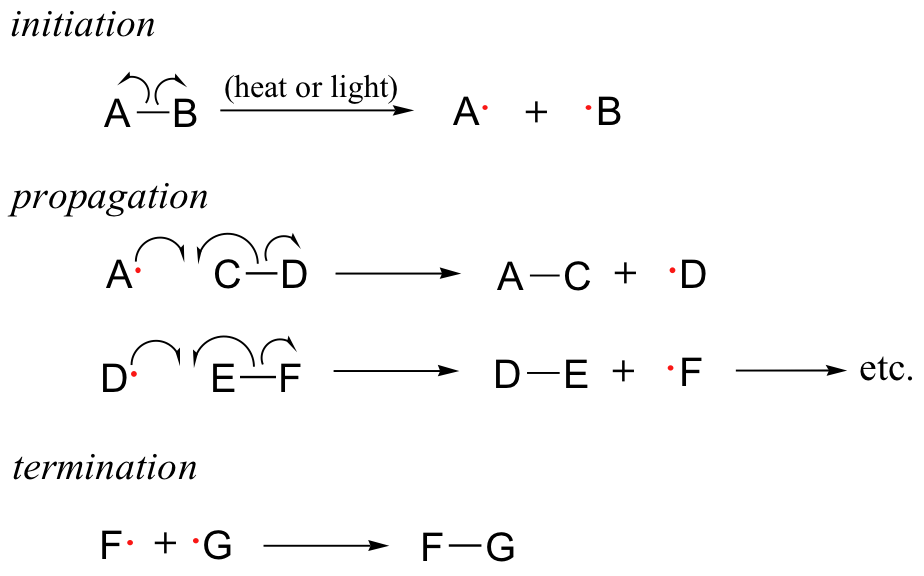
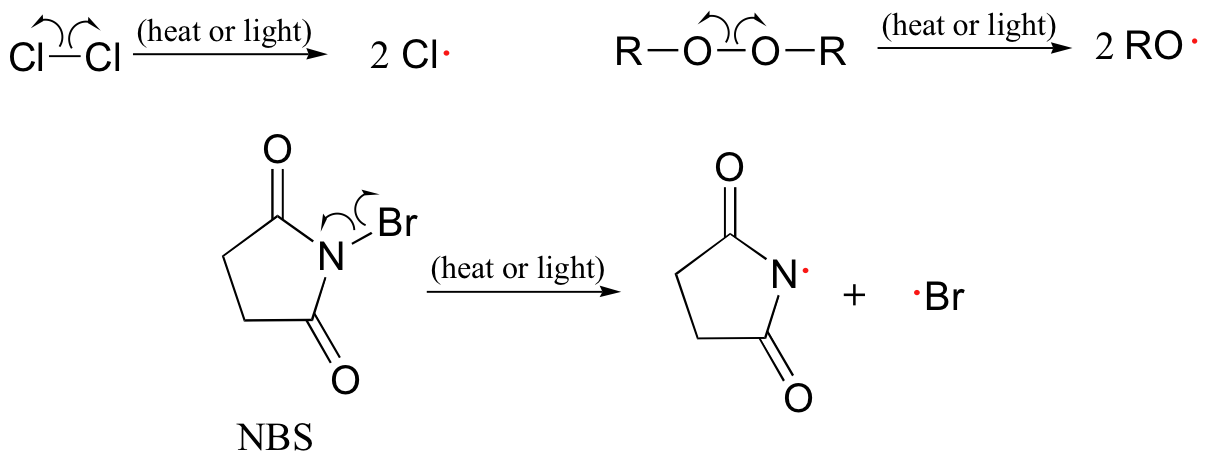
The initiation phase describes the step that initially creates a radical species. In most cases, this is a homolytic cleavage event, and takes place very rarely due to the high energy barriers involved. Often the influence of heat, UV radiation, or a metal-containing catalyst is necessary to overcome the energy barrier.
Molecular chlorine and bromine will both undergo homolytic cleavage to form radicals when subjected to heat or light. Other functional groups which also tend to form radicals when exposed to heat or light are chlorofluorocarbons, peroxides, and the halogenated amide N-bromosuccinimide (NBS).
The propagation phase describes the 'chain' part of chain reactions. Once a reactive free radical is generated, it can react with stable molecules to form new free radicals. These new free radicals go on to generate yet more free radicals, and so on. Propagation steps often involve hydrogen abstraction or addition of the radical to double bonds.
Chain termination occurs when two free radical species react with each other to form a stable, non-radical adduct. Although this is a very thermodynamically downhill event, it is also very rare due to the low concentration of radical species and the small likelihood of two radicals colliding with one another. In other words, the Gibbs free energy barrier is very high for this reaction, mostly due to entropic rather than enthalpic considerations. The active sites of enzymes, of course, can evolve to overcome this entropic barrier by positioning two radical intermediates adjacent to one another.
17.2B: Radical halogenation in the lab
The chlorination of an alkane provides a simple example of a free radical chain reaction. In the initiation phase, a chlorine molecule undergoes homolytic cleavage after absorbing energy from light:
![]()
The chlorine radical then abstracts a hydrogen, leading to an alkyl radical (step 2), which reacts with a second chlorine molecule (step 3) to form the chloroalkane product plus chlorine radical, which then returns to repeat step 2.
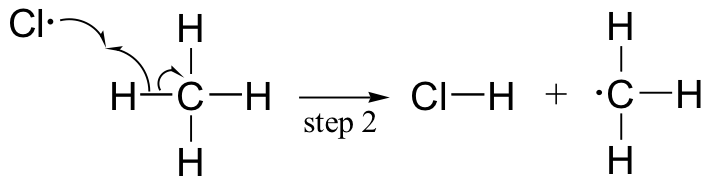
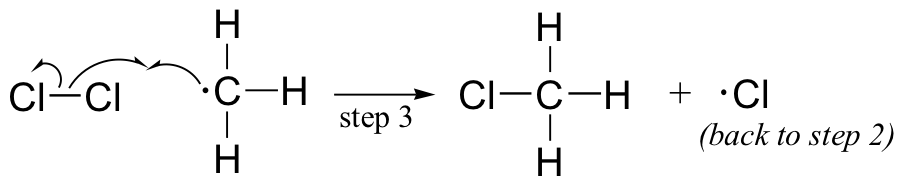
Likely chain termination steps are the condensation of two alkyl radical intermediates or condensation of an alkane radical with a chlorine radical.
Alkane halogenation reactions exhibit a degree of regiospecificity: if 2-methylbutane is subjected to a limiting amount of chlorine, for example, chlorination takes place fastest at the tertiary carbon.
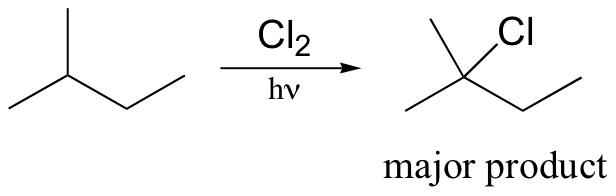
This is because the tertiary radical intermediate is more stable than the secondary radical intermediate that results from abstraction of the proton on carbon #3, and of course both are more stable than a primary radical intermediate. Recall that the Hammond postulate (section 6.2, section 15.2B) tells us that a lower-energy intermediate implies a lower-energy transition state, and thus a faster reaction.
Unfortunately, chloroalkanes will readily undergo further chlorination resulting in polychlorinated products, so this is not generally a terribly useful reaction from a synthetic standpoint.
Alkanes can be brominated by a similar reaction. The regiochemical trends are the same as for chlorination, but significantly more pronounced (in other words, bromination is more regioselective). This is because hydrogen abstraction by bromine radical is much less exergonic than by chorine radical – and this in turn means that the transition state for abstraction by bromine resembles the resulting intermediate more closely than the transition state for abstraction by chlorine resembles its intermediate.
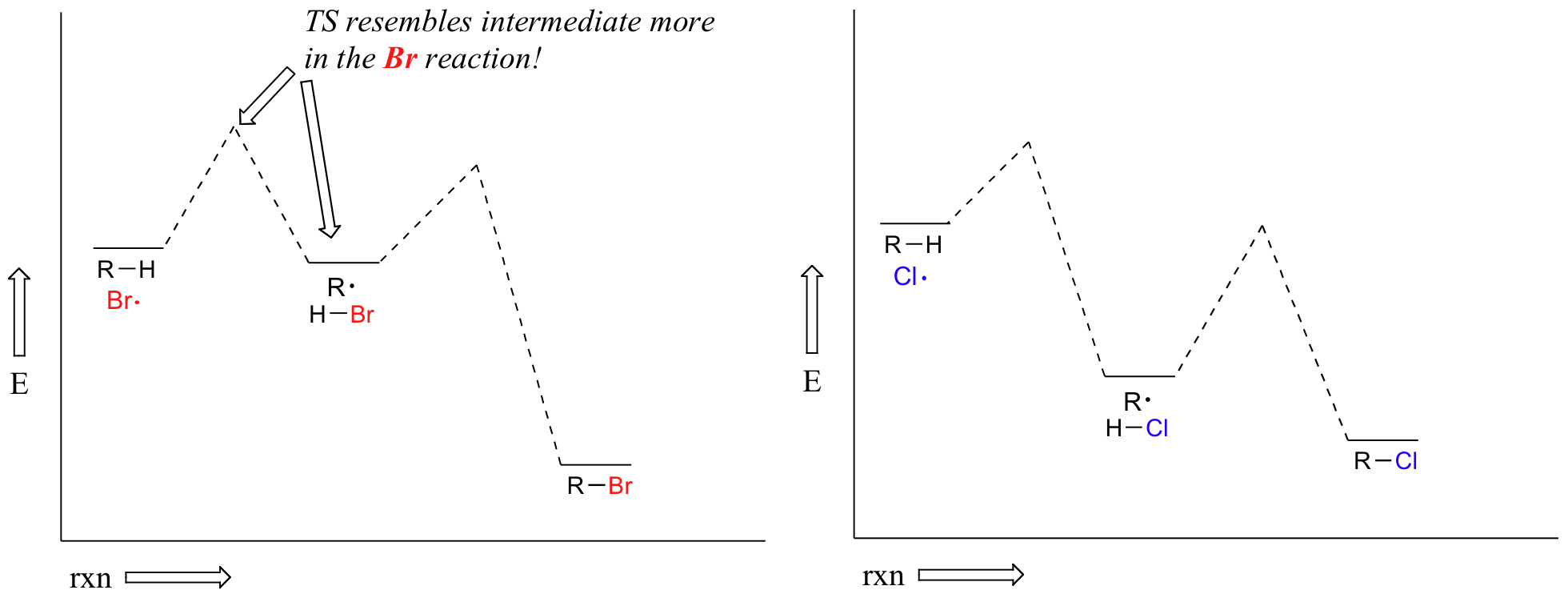
Another way of saying the same thing is that the bromination transition state has more ‘radical character’ than the chlorination transition state. Trends in radical stability thus have a greater influence on the speed of hydrogen abstraction.
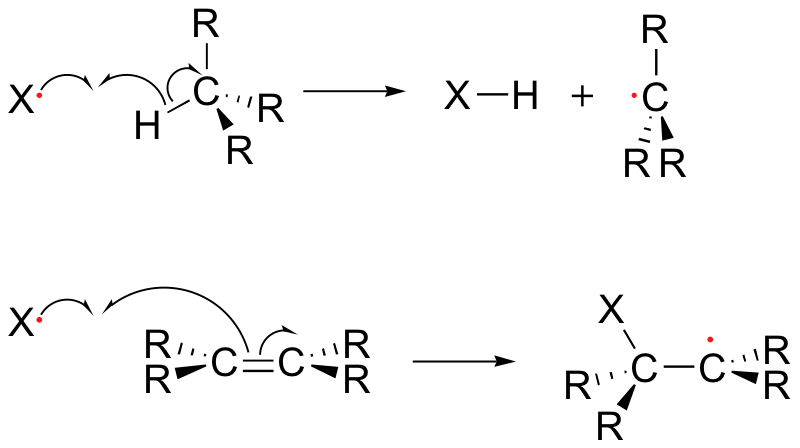
Alkenes and alkylbenzene can be halogenated with high regiospecificity using N-bromosuccinimide (NBS) in the presence of light or a radical initiator such as benzoyl peroxide. Bromination takes place specifically at the allylic position of alkenes and at the benzylic position of alkylbenzene (recall from section 17.1A that allylic and benzylic radicals are stabilize by resonance).
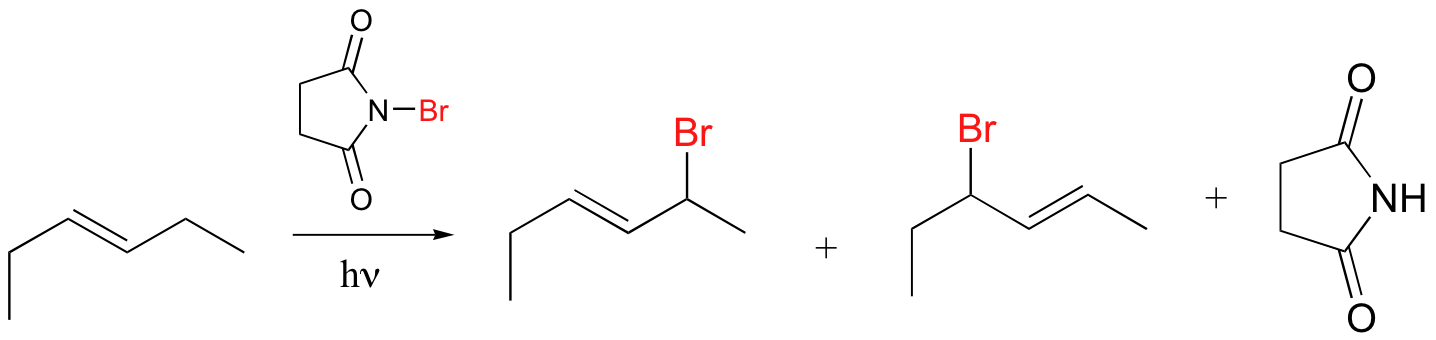
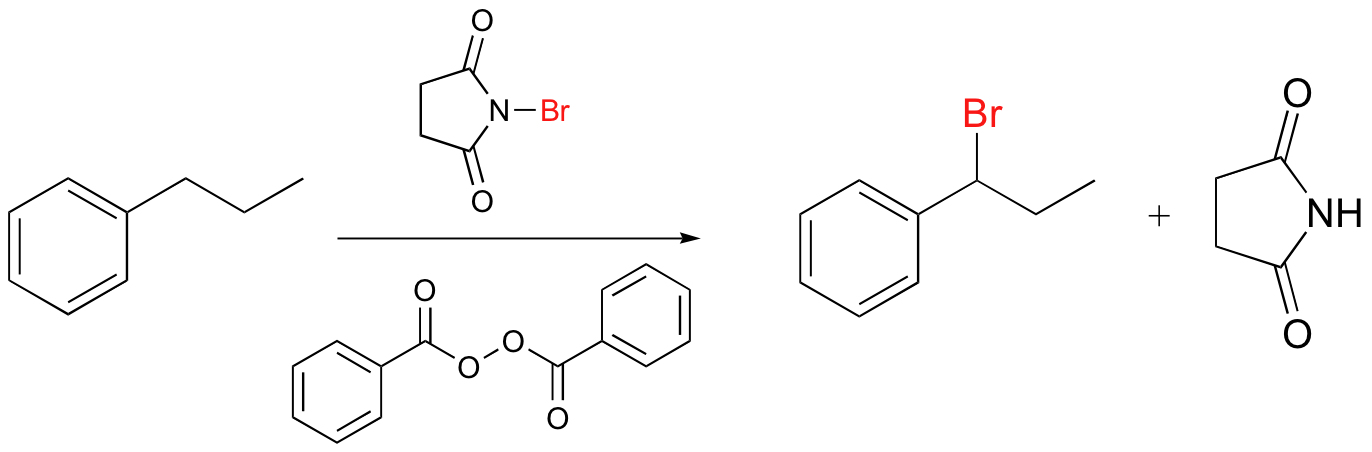
A mechanism for allylic bromination by NBS is shown below:
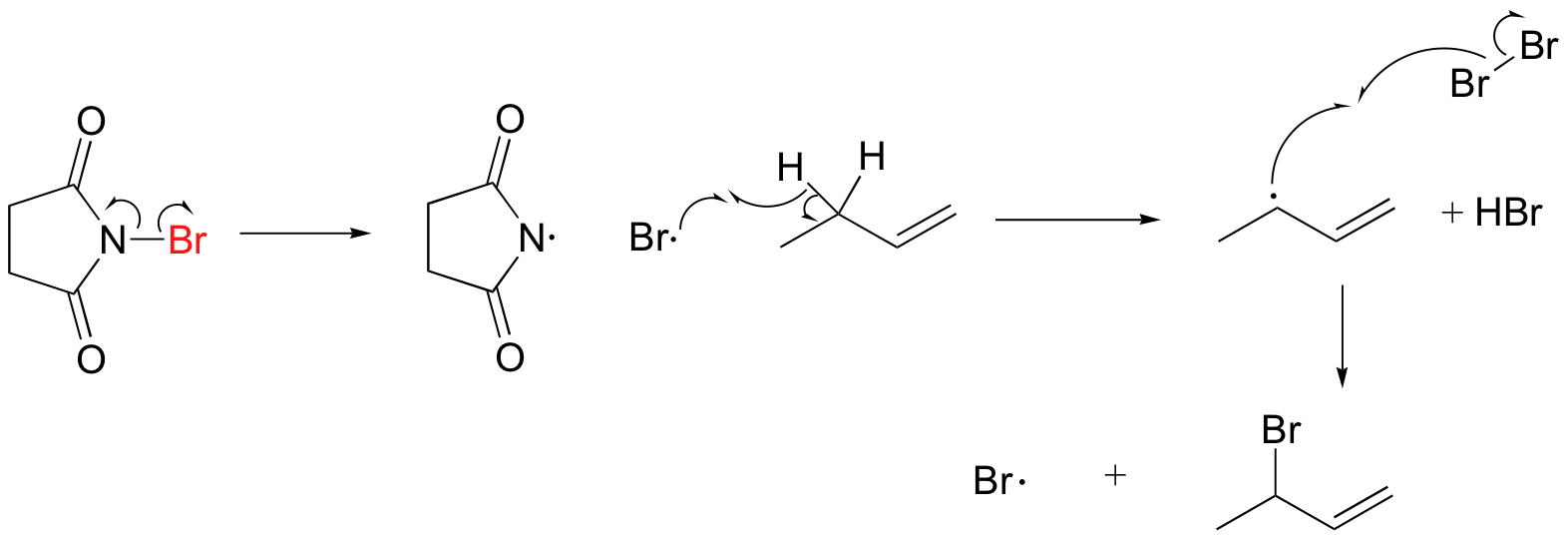
The Br2 in this process is formed in a side reaction between HBr and NBS (not shown).
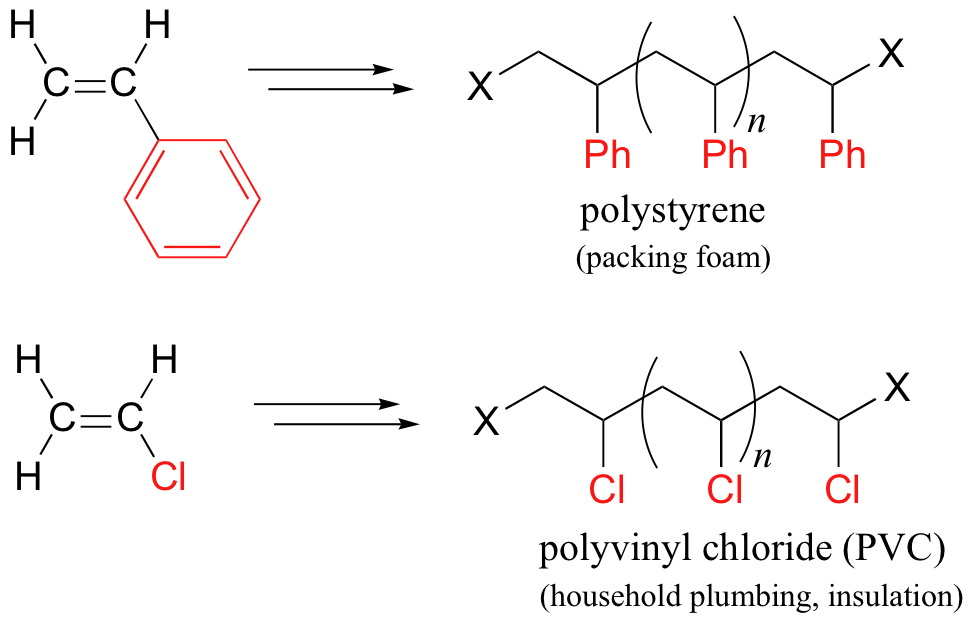
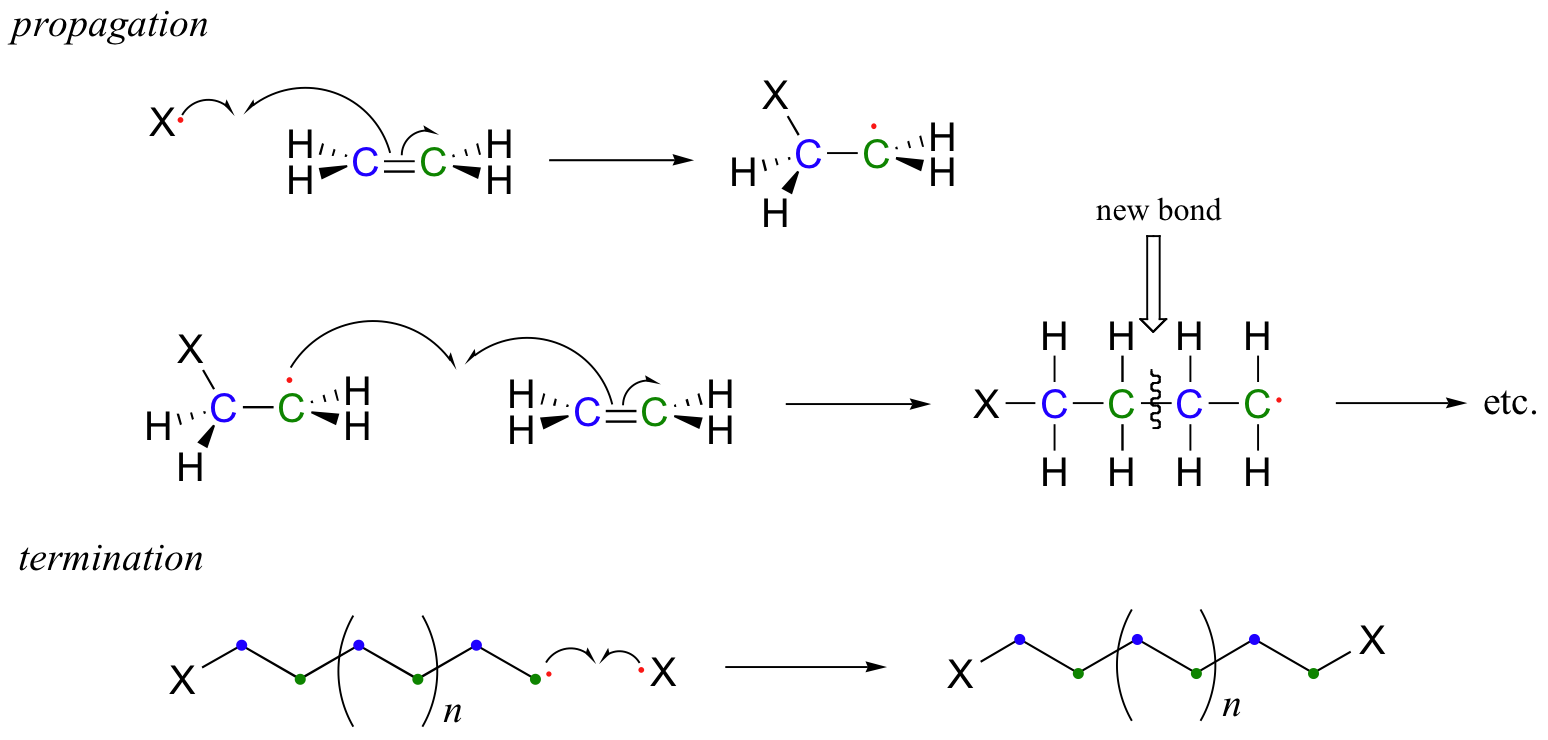
17.2C: Useful polymers formed by nonenzymatic radical chain reactions
Many household polymeric materials with which you are probably familiar are made with a radical chain reaction process. Polyethylene (PET), the plastic material used to make soft drink bottles and many other kinds of packaging, is produced by the radical polymerization of ethylene (ethene in IUPAC nomenclature). A radical initiator such as benzoyl peroxide undergoes homolytic cleavage when subjected to high temperatures.
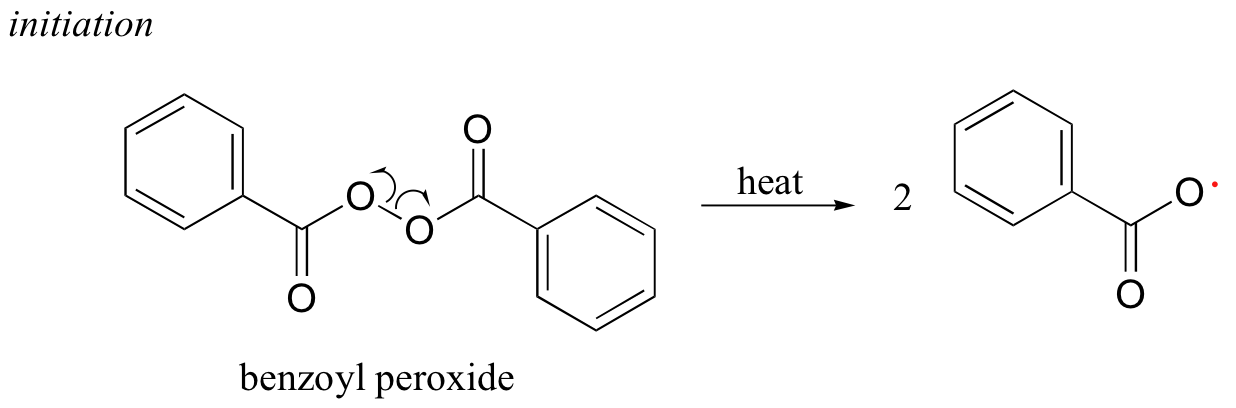
In the propagation phase, the benzoyl radical (X• in the figure below) adds to the double bond of ethylene, generating a new organic radical. Successive ethylene molecules add to the growing polymer, until termination occurs when two radicals happen to collide. In the figure below, the growing PET polymer is terminated by a benzoyl radical, but in an alternative termination step two growing PET radicals could condense.
Other small substituted alkene monomers polymerize in a similar fashion to form familiar polymer materials. Two examples are given below.