3.7: Meso compounds
- Page ID
- 1045
The levorotatory and dextrorotatory forms of tartaric acid studied by Louis Pasteur were, as we now know, the (S,S) and (R,R) enantiomers, respectively:
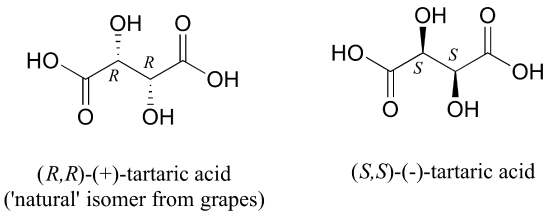
What the 19th century chemists referred to as 'acide racemique' was just that: a racemic mixture of the R,R and S,S enantiomers, the racemization a result of how the natural R,R isomer had been processed.
But tartaric acid has two chiral centers: shouldn't there be another pair of enantiomers?
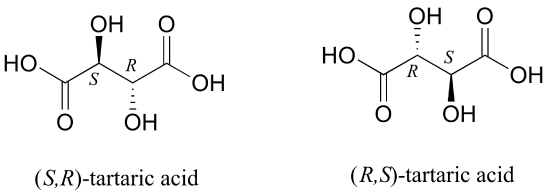
There in fact is another stereoisomer of tartaric acid: but only one. The two structures above are actually superimposable on one another: they are the exact same molecule. The figure below illustrates this, and also that the structure has a plane of symmetry. However, you should be sure to build models and confirm these assertions for yourself.
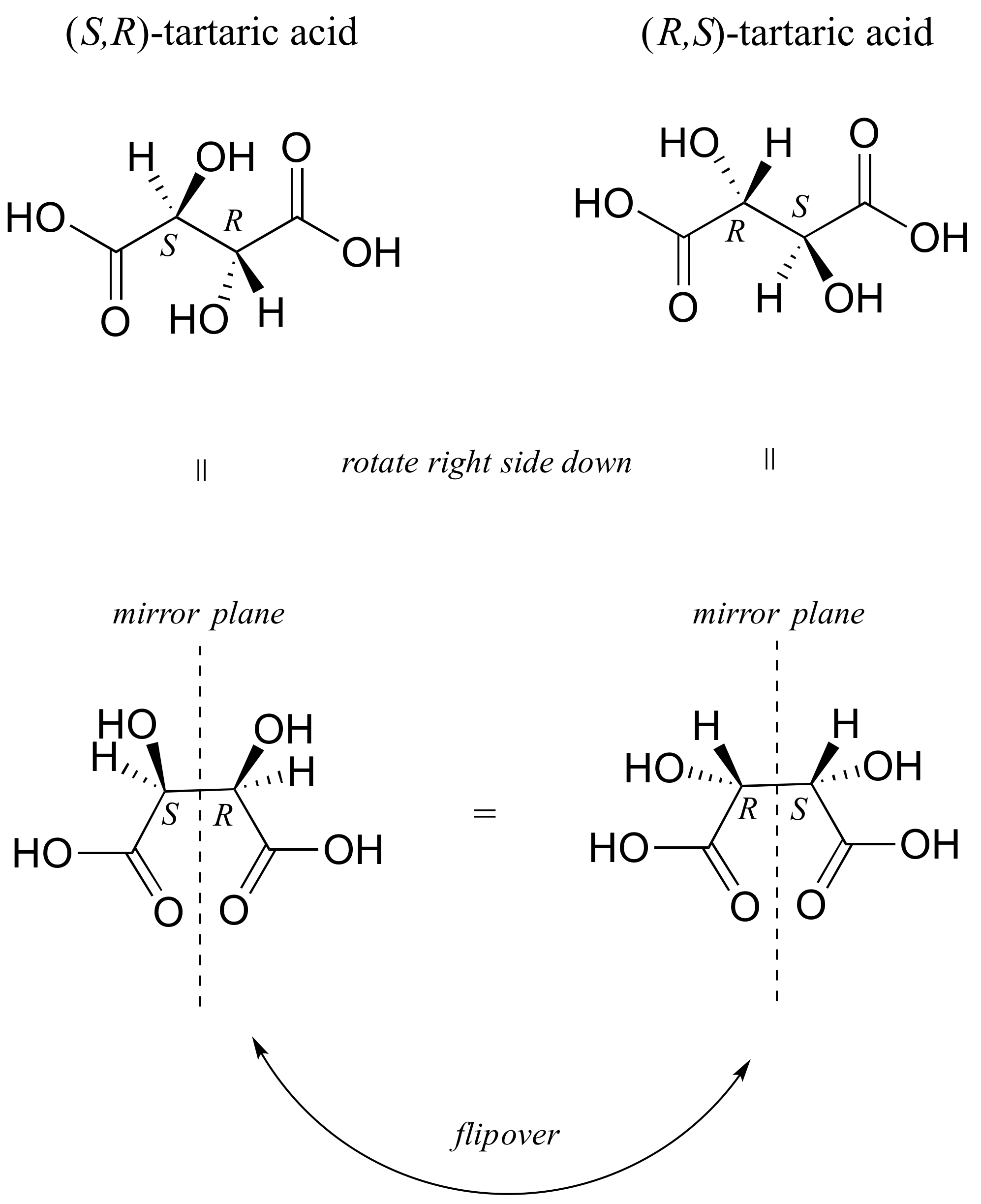
This tartaric acid isomer is an achiral diastereomer of the both the levorotatory and dextrorotatory isomers. It is a special case, called a meso compound: it has two apparent chiral centers but due to its internal symmetry it is not in fact chiral, and does not exhibit optical activity. Note that the meso form of tartaric acid did not play a part in Pasteur's experiments.
There are many more possible examples of meso compounds, but they really can be considered 'exceptions to the rule' and quite rare in biologically relevant chemistry.
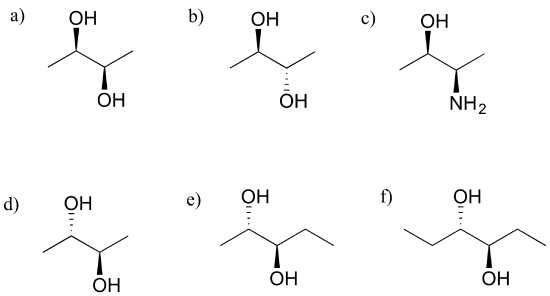
Exercise 3.23: Which of the following compounds are meso? Hint: build models, and then try to find a conformation in which you can see a plane of symmetry.
Kahn Academy video tutorial on meso compounds


