3.10: Stereochemistry in biology and medicine
- Page ID
- 1047
While challenging to understand and visualize, the stereochemistry concepts we have explored in this chapter are integral to the study of living things. The vast majority of biological molecules contain chiral centers and/or stereogenic alkene groups. Most importantly, proteins are chiral, which of course includes all of the the enzymes which catalyze the chemical reactions of a cell, the receptors which transmit information within or between cells, and the antibodies which bind specifically to potentially harmful invaders. You know from your biology classes that proteins, because they fold up into a specific three dimensional shape, are able to very specifically recognize and bind to other organic molecules. The ligand or substrate bound by a particular protein could be a small organic molecule such as pyruvate all the way up to a large biopolymer such as a specific region of DNA, RNA, or another protein. Because they are chiral molecules, proteins are very sensitive to the stereochemistry of their ligands: a protein may bind specifically to (R)-glyceraldehyde, for example, but not bind to (S)-glyceraldehyde, just as your right hand will not fit into a left-handed baseball glove (see end of chapter for a link to an animation illustrating this concept).
The over-the-counter painkiller ibuprofen is currently sold as a racemic mixture, but only the S enantiomer is effective, due to the specific way it is able to bind to and inhibit the action of prostaglandin H2 synthase, an enzyme in the body's inflammation response process.
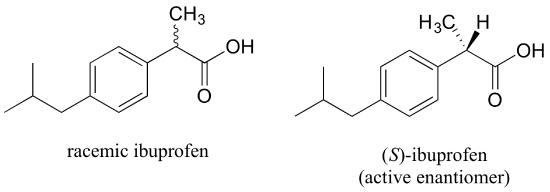
The R enantiomer of ibuprofen does not bind to prostaglandin H2 synthase in the same way as the S enantiomer, and as a consequence does not exert the same inhibitory effect on the enzyme's action (see Nature Chemical Biology 2011, 7, 803 for more details). Fortunately, (R)-ibuprofen apparently does not cause any harmful side effects, and is in fact isomerized gradually by an enzyme in the body to (S)-ibuprofen.
Earlier in this chapter we discussed the tragic case of thalidomide, and mentioned that it appears that it is specifically the S enantiomer which caused birth defects. Many different proposals have been made over the past decades to try to explain the teratogenic (birth defect-causing) effect of the drug, but a clear understanding still evades the scientific community. In 2010, however, a team in Japan reported evidence that thalidomide binds specifically to a protein called 'thereblon'. Furthermore, when production of thereblon is blocked in female zebra fish, developmental defects occur in her offspring which are very similar to the defects caused by the administration of thalidomide, pointing to the likelihood that thalidomide binding somehow inactivates the protein, thus initiating the teratogenic effect. (http://news.sciencemag.org/2010/03/t...-partner-crime)
You can, with a quick trip to the grocery store, directly experience the biological importance of stereoisomerism. Carvone is a chiral, plant-derived molecule that contributes to the smell of spearmint in the R form and caraway (a spice) in the S form.
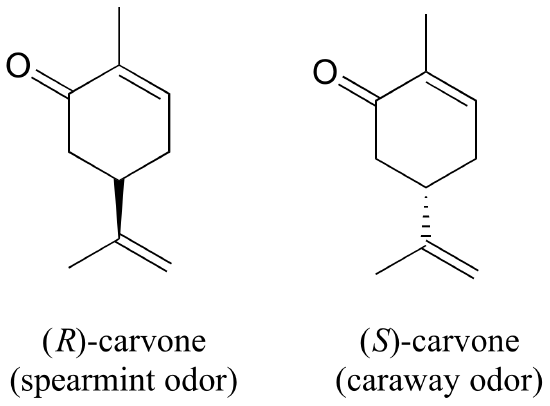
Although details are not known, the two enantiomers presumably interact differently with one or more smell receptor proteins in your nose, generating the transmission of different chemical signals to the olfactory center of your brain.
Exercise 3.28: Ephedrine, found in the Chinese traditional medicine ma huang, is a stimulant and appetite suppressant. Both pseudoephedrine and levomethamphetamine are active ingredients in over-the-counter nasal decongestants. Methamphetamine is a highly addictive and illegal stimulant, and is usually prepared in illicit 'meth labs' using pseudoephedrine as a starting point.
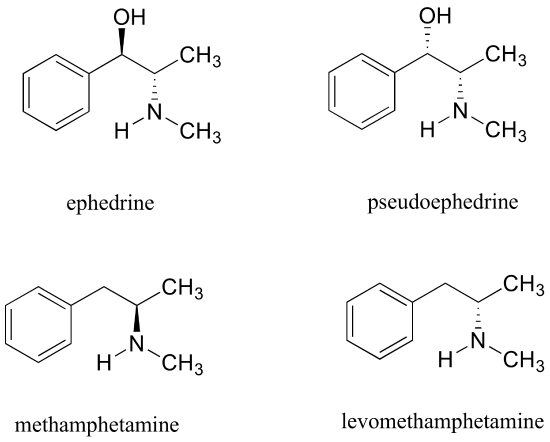
What is the relationship between ephedrine and pseudoephedrine? Between methamphetamine and levomethamphetamine? Between pseudoephedrine and methamphetamine? Your choices are: not isomers, constitutional isomers, diastereomers, enantiomers, or same molecule.
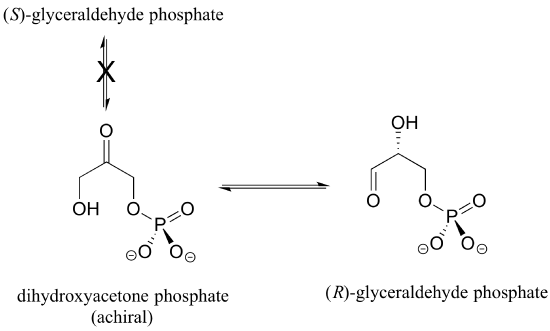
Enzymes are very specific with regard to the stereochemistry of the reactions they catalyze. When the product of a biochemical reaction contains a chiral center or a stereogenic alkene, with very few exceptions only one stereoisomer of the product is formed. In the glycolysis pathway, for example, the enzyme triose-phosphate isomerase catalyzes the reversible interconversion between dihydroxyacetone (which is achiral) and (R)-glyceraldehyde phosphate. The (S)-glyceraldehyde enantiomer is not formed by this enzyme in the left-to-right reaction, and is not used as a starting compound in the right-to-left reaction - it does not 'fit' in the active site of the enzyme.
In the isoprenoid biosynthesis pathway, two five-carbon building-block molecules combine to form a ten-carbon chain containing an E-alkene group. The enzyme does not catalyze formation of the Z diastereomer.
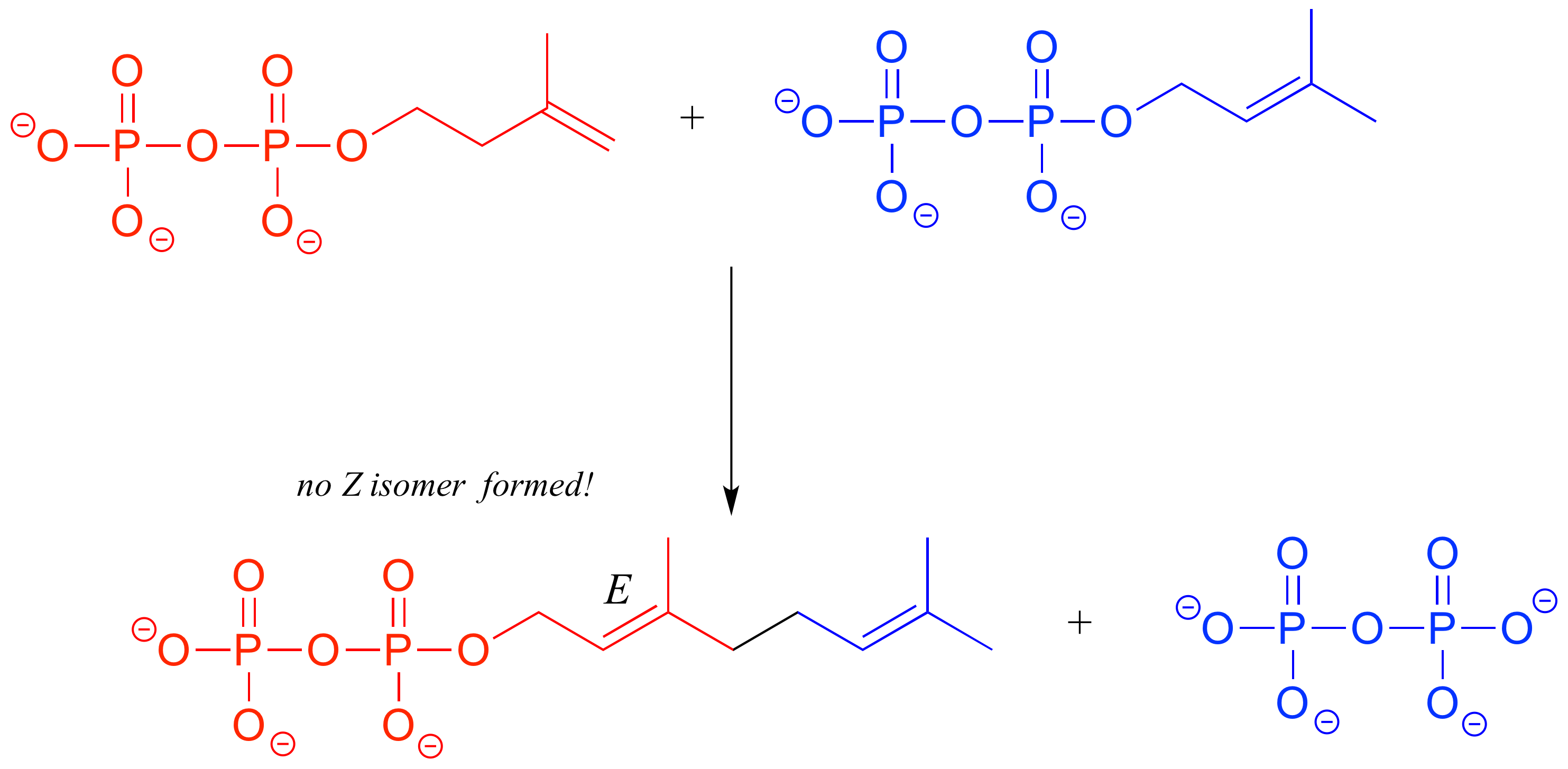
In chapters 9-17 of this book, and continuing on into your study of biological and organic chemistry, you will be learning about how enzymes are able to achieve these feats of stereochemical specificity. If you take a more advanced class in organic synthesis, you will also learn how laboratory chemists are figuring out ingenious ways to exert control over the stereochemical outcomes of nonenzymatic reactions, an area of chemistry that is particularly important in the pharmaceutical industry.


