7: Acids and bases
- Page ID
- 21355
A complete PDF version of Organic Chemistry With a Biological Emphasis is available here as a free download.
Introduction to Chapter 7
A foul brew that spread light on an age-old disease
Helicobacter pylori
(Photo Credit: AJ Cann, flickr)
The glass flask sitting on a bench in Dr. Barry Marshall's lab in Perth, Western Australia, contained about thirty milliliters of a distinctly unappetizing murky, stinking yellowish liquid.
A few days earlier, Dr. Marshall, in consultation with his mentor and research partner Dr. J. Robin Warren, had poured a nutrient broth into the flask, then dropped in a small piece of tissue sample taken from the stomach of a patient suffering from chronic gastritis. Gastritis is an inflammation of the stomach lining characterized by lingering pain, nausea, and vomiting, and is often is the precursor to a peptic ulcer. Now, after several days of incubating in a warm water bath, the flask contained a living liquid culture, swarming with billions of bacterial cells. According to the medical textbooks lining Dr. Marshall's bookshelves, the bacteria should not have been there – nothing should have grown in the broth, because the highly acidic environment inside the stomach was supposed to be sterile. Also according to the textbooks, the cause of his patient's stomach ailment was stress, or perhaps a poor diet – but most definitely not a bacterial infection.
Dr. Marshall took a good, long, look at the contents of the flask. Then he gave it a final swirl, and drank it down.
Five days later, his stomach started to hurt.
This is the story of how two doctors dared to think what no one else had thought, and turned an established medical doctrine upside-down. Dr. J. Robin Warren was a pathologist at the Royal Perth Hospital in Perth, Western Australia. One day, when examining an image taken from a stomach biopsy from a patient with severe gastritis, he noticed what appeared to be spiral-shaped bacteria in the tissue, a surprising observation given the medical consensus that bacteria could not live in the stomach.
The microbe-like shapes were very hard to see, but when he tried treating the sample with a silver stain they became much more apparent. He decided to start looking at silver-stained sections of every stomach biopsy he examined, and before long he noticed a pattern: the presence of the spiral bacteria coincided with gastritis in the patient, and the tissues which were more severely inflamed seemed also to have more bacteria present. Could there be a causal link between the bacteria and the illness?
When he discussed his observations with colleagues, they dismissed the results as coincidental contamination – nothing important.
Warren wasn't willing to just let it go. He was able to interest Barry Marshall, a young internal medicine resident training at the same hospital, in taking on a research project to try and solve the mystery of the bacteria that were not supposed to exist. Marshall started by doing a thorough search of the literature, and found that his mentor was not the first to report seeing spiral-shaped bacteria in stomach tissue – there were in fact several such observations in the literature, the oldest going back to the middle of the 19th century. All of them had been dismissed as unimportant artifacts.
Warren and Marshall started sending stomach biopsies from gastritis patients to the microbiology lab in the hospital, to see if bacteria from the samples could be cultured in a petri dish. For many months, they got nothing. Then one Tuesday morning, right after the four-day Easter holiday, Marshall got a call from an excited microbiology technician: he had neglected to dispose of the latest round of test cultures before going home for the holiday, and instead had left them in the incubator the whole time. After growing for a full five days, the dishes had colonies of bacteria growing in them. All this time, the technicians had been throwing the cultures away after two days when no bacterial growth was evident – standard procedure when working with other bacteria – but apparently these bacteria were especially slow-growing.
Now that he could culture the bacteria in the lab, he was able to isolate and study them in more detail, and gave them the name Helicobacter pylori. Marshall confirmed Warren's earlier observation of a correlation between stomach bacteria and gastritis: only those patients suffering from gastritis or ulcers seemed to have the bacteria in their stomachs. There seemed to be strong evidence that H. pylori infection led to gastritis, which in turn lead eventually to stomach ulcers.
Despite the new data, Warren and Marshall's colleagues in the gastroenterology field were still unconvinced of the link between bacteria and gastritis or ulcers. Scientists are inherently skeptical people, and it was difficult to overcome the long-entrenched theory that the stomach was sterile, and that ulcers were caused by stress. Interviewed much later by Discovery Magazine, Marshall recalled: “To gastroenterologists, the concept of a germ causing ulcers was like saying that the Earth is flat”.
Poring once more over the available literature, Marshall learned that acid-reducing drugs – an enormously profitable product– were able to relieve ulcer symptoms, but only temporarily. One interesting piece of information stood out to him, though: an over-the-counter antacid containing the element bismuth (similar the brand-name medicine Pepto-Bismol) provided much longer-lasting relief compared to the other drugs – and in some cases seemed to effect a permanent cure. Marshall soaked a small circle of filter paper in the bismuth medicine, and placed it on a petri dish that he had inoculated with H. pylori. After five days in the incubator, there was a clear circle of non-growth around the filter paper. The medicine had killed the bacteria.
Everything seemed to fit together: almost all patients with gastritis or ulcers had H. pylori infections, and a drug which was able to kill the bacteria was also effective against the stomach ailment. But to convince the medical community that the root cause of ulcers was H. pylori infection, Warren and Marshall needed more direct evidence: they had to show that a healthy stomach – free from gastritis and uninfected by H. pylori – would develop gastritis as a result of intentional infection, and that clearing up the infection would also cure the gastritis. They tried experiments with pigs first, then rats, and then mice, but to no avail – they were not able to induce an H. pylori infection in the animal models. Marshall was getting desperate: all around him he saw patients suffering terribly, getting only temporary relief from acid blockers and eventually needing to have parts of their stomachs removed, and he was convinced that simple antibiotic therapy would cure them if only their doctors could be convinced to try it. Because animal models had failed, he decided to move to a model system that he knew would work: humans. Ethical and regulatory considerations prevented him from intentionally infecting human volunteers - so his only option was to use himself as a guinea pig.
We are now at the point in the story where Barry Marshall, in the name of science and medicine, took his disgusting but undeniably courageous gulp of bacteria-laden broth. He had already undergone an endoscopy to ascertain that his stomach was free from both inflammation and H. pylori. As we already know, he started to develop symptoms of gastritis about five days after drinking the bacteria – the same amount of time that it took for H. pylori colonies to appear in the petri dish cultures. After a few more days, he underwent another endoscopy, and was overjoyed to be told that his stomach was indeed inflamed, and was infested with spiral-shaped bacteria. He initially wanted to carry on the experiment for a few more days of further tests, but his wife had a different opinion on the matter and convinced him to begin antibiotic treatment, which quickly cleared up both his infection and his stomach inflammation.
Marshall and Warren now had clear, direct evidence that the stomach inflammation which leads to ulcers was caused by bacteria, and could be cured with antibiotics. They submitted a summary of their results for presentation at a meeting of the Gastroenterological Society of Australia, but were rejected. Apparently, 67 submissions had come in and there was only time for 56 presentations; unfortunately their results were not considered important enough to make the cut. They persevered, and eventually published their findings in the June, 1984 issue of the British medical journal The Lancet.
The paper gained some notice, especially from microbiologists, but did not have an immediate impact in clinical practice. Around the world, gastroenterologists continued to treat ulcers with acid blockers. Outside of the mainstream medical community, however, word was getting out that two Australian doctors had a cure for ulcers, and more people started coming to them for treatment. Stories about Warren and Marshall appeared in places like Reader's Digest and The National Enquirer, and eventually in the United States the National Institutes of Health and the Food and Drug Administration responded by fast-tracking the clinical testing and approval process, and publicizing the new treatment option.
Stomach ulcers, which have been tormenting human beings since the beginning of recorded history, are today considered an easily curable condition, and the idea that they are caused by H. pylori infection is fully accepted by the medical community. Drs. J. Robin Warren and Barry Marshall shared the 2005 Nobel Prize in Medicine.
The now-discredited idea that the stomach is a sterile place made perfectly good biochemical sense at the time: the stomach is like a bathtub full of hydrochloric acid, which you probably recall from previous chemistry classes and labs is a very strong, dangerously corrosive acid. It is very difficult to imagine how a microbe could survive in such an environment. We now know that H. pylori can thrive there in part because its spiral shape allows it to burrow deep into the protective layer of mucus that coats the stomach wall. In addition, H. pylori cells produce large amounts of an enzyme called urease, which catalyzes a reaction between urea and water to form carbon dioxide and ammonia:
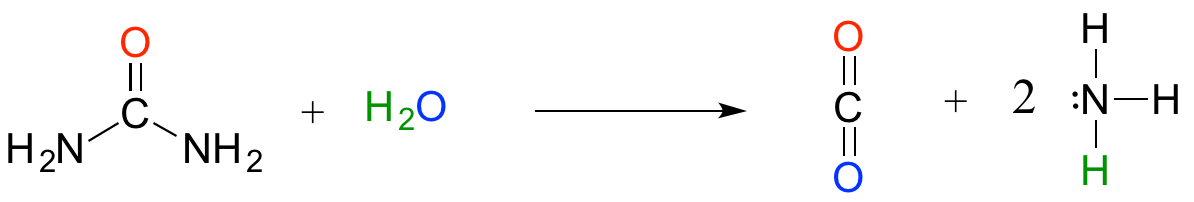
Ammonia - the main component of window-washing liquid - is a fairly strong base, and reacts rapidly and completely with hydrochloric acid to neutralize it.
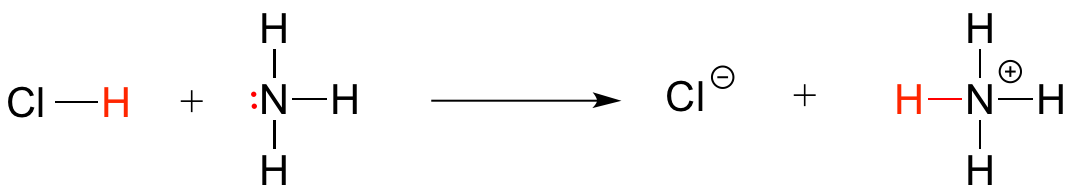
Although scientists are still unsure of all the details, it is likely that these two protective strategies used by H. pylori somehow play a role in causing the inflammation that can lead to peptic ulcers, where the stomach lining becomes exposed to the harsh action of hydrochloric acid.
The idea of acidity is at the heart of our story about the discovery of H. pylori, and is the subject of this chapter. From here on in our study of organic chemistry, we will be learning about how organic molecules react, and how their structure determines their reactivity. The reaction between an acid and a base - where a proton is donated from the former and accepted by the latter - is the first kind of organic reaction that we will explore. After reviewing some basic ideas about acid-base equilibria with which you are probably already familiar from General Chemistry, we will dive into some very challenging new waters, as we attempt to use our understanding of organic structure to predict how different organic functional groups will react in an acid-base context. Many of the ideas that are introduced in this chapter, though perhaps difficult to grasp at first, will be crucial to understanding not only acid base chemistry but all of the other organic reaction types that we will see throughout the remainder of the book.
Additional reading: Discover Magazine, March 7, 2010. The Doctor Who Drank Infectious Broth, Gave Himself an Ulcer, and Solved a Medical Mystery


