Appendix: Review of laboratory synthesis reactions
- Page ID
- 1125
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
While the focus of this textbook is on organic reactions occuring in living cells, if you are a chemistry major, or are planning to take a standardized exam such as the MCAT, you will need to be familiar with a number of laboratory synthesis reactions. Here, we review the lab synthesis reactions covered in this text, which include most of the reactions typically covered in traditional organic texts. Click on the chapter/section number for direct links to the section where these reactions are introduced.

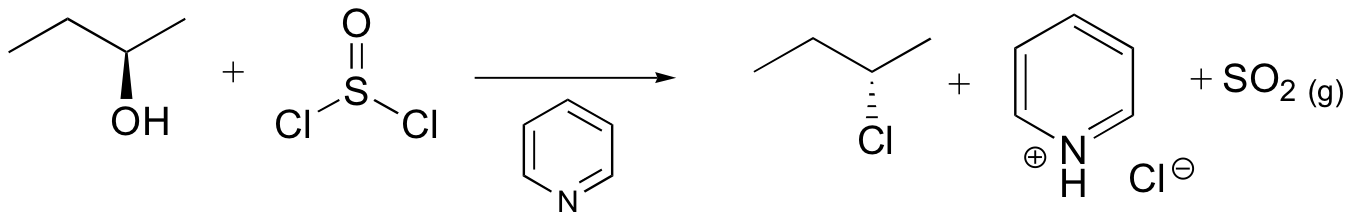
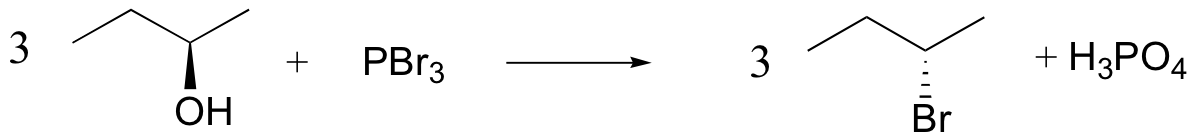
alcohols converted into good leaving groups
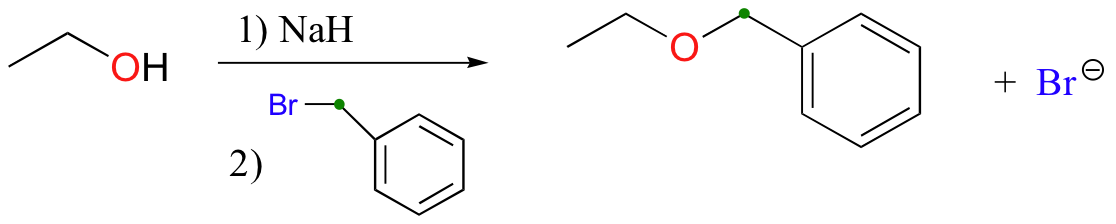
Williamson ether synthesis
alkyl halide must be methyl or primary to avoid competing elimination
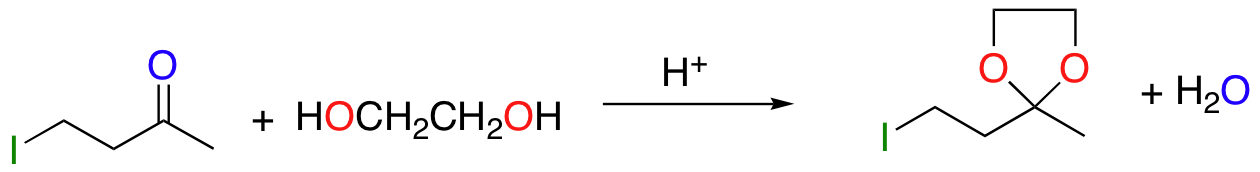
cyclic acetal ‘protects’ ketone/aldehyde group – stable to bases/nucleophiles
deprotect with aqueous acid
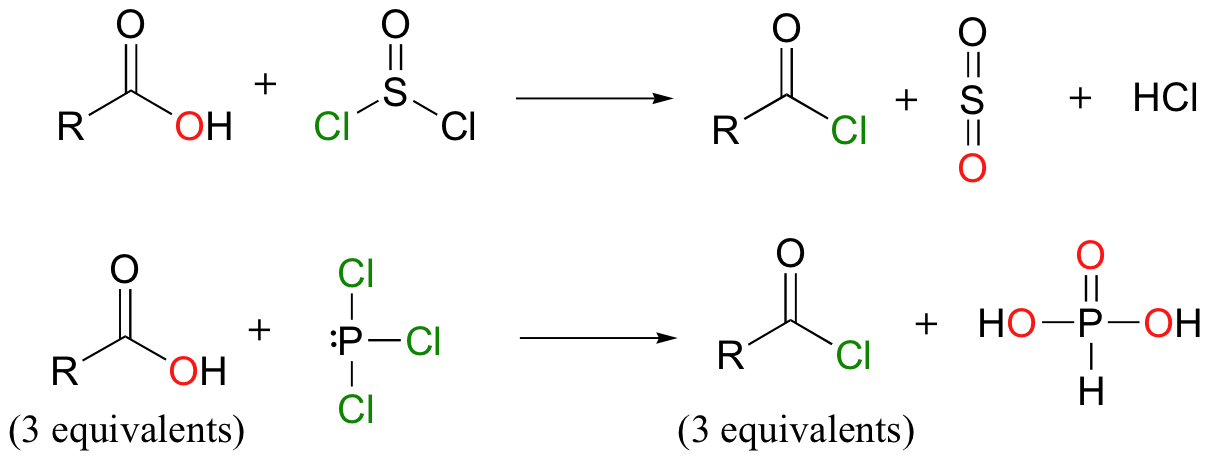
activates carboxylic acids
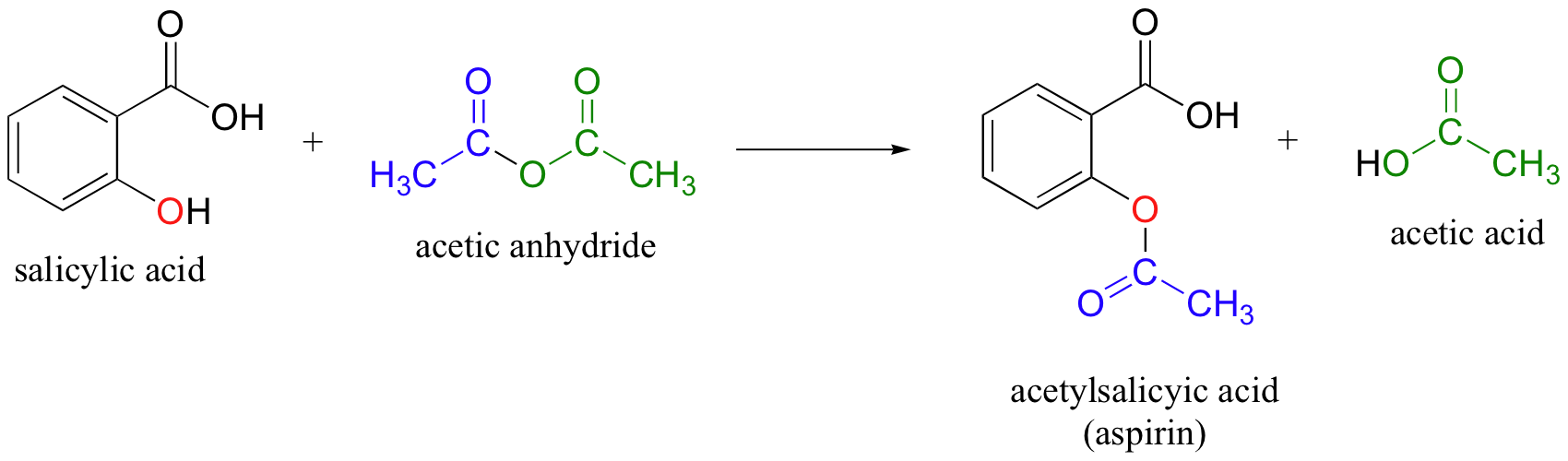
acetic anhydride is a good acetyl group donor (activated acetic acid)
adds acetyl group to acohols, amines
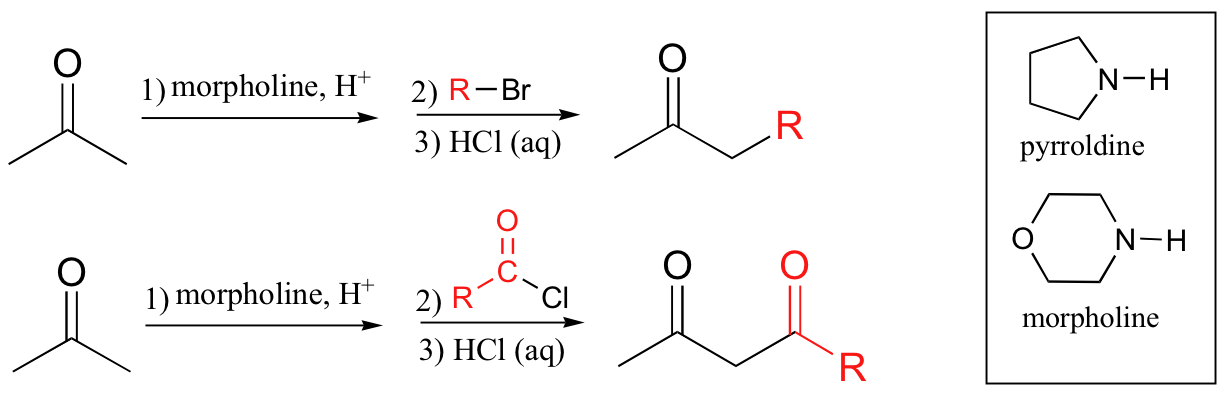
goes through enamine intermediate
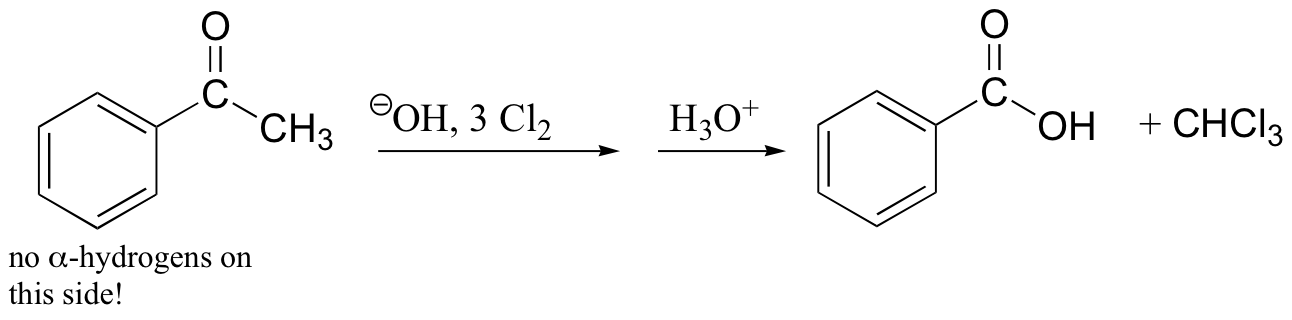
haloform reaction – also works with Br2, I2
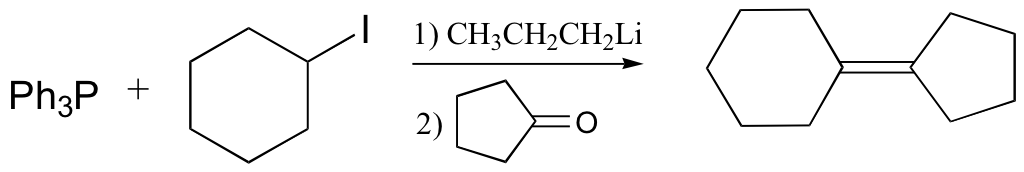
Wittig reaction
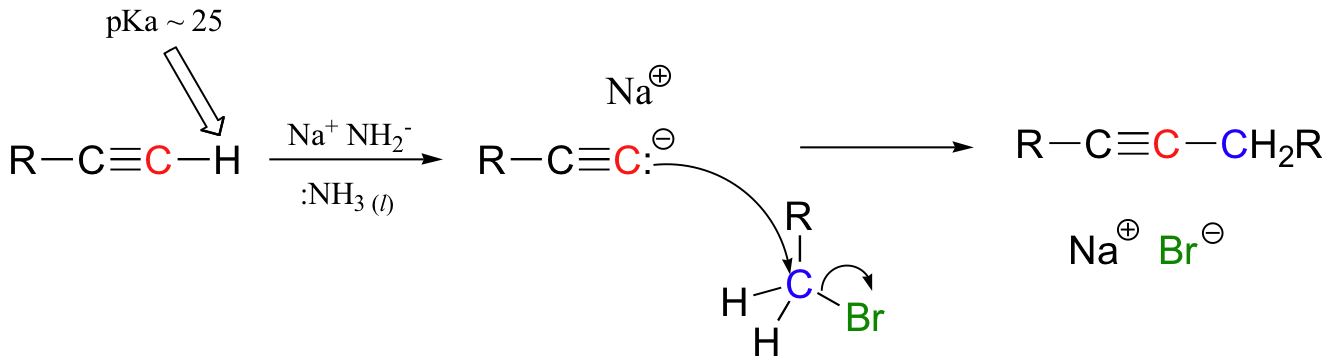
Grignard reagent – carbon nucleophile
No acidic protons can be present (it’s a strong base)
Can also use R-Cl
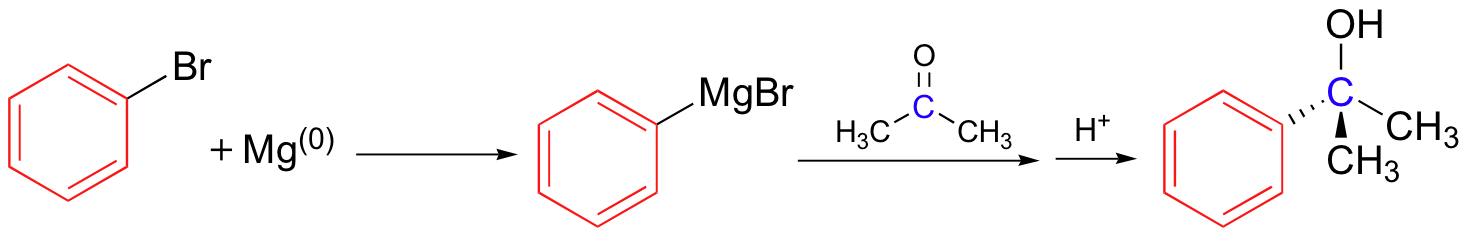
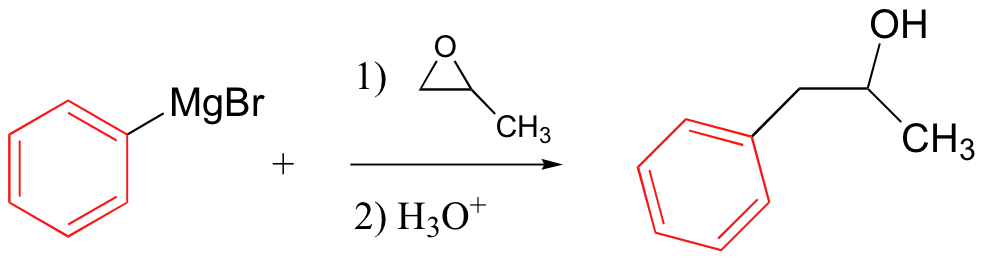
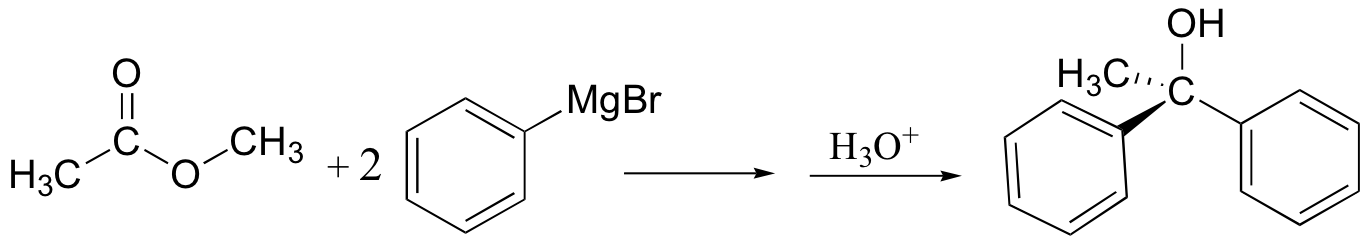
Grignards add to esters, acid chlorides twice
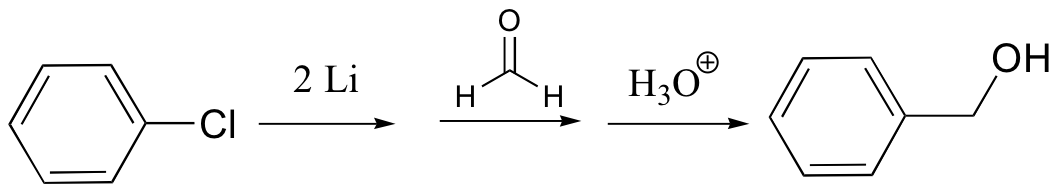
organolithium – similar to Grignard
![]()
Gilman reagent
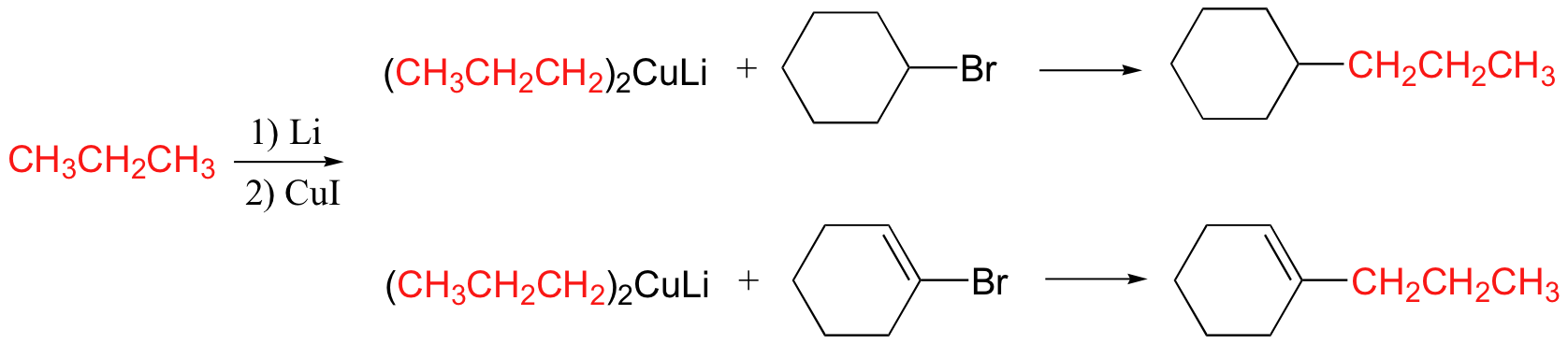
Gilman reagent will react with alkyl, vinyl halides as well as carbonyls
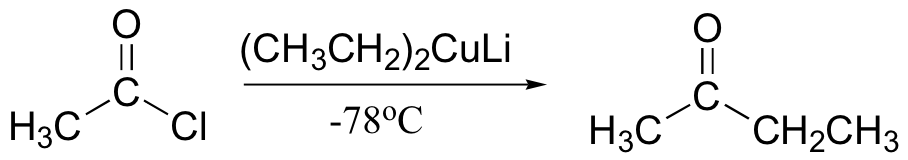
Gilman reagent will add once to acid chlorides to make a ketone
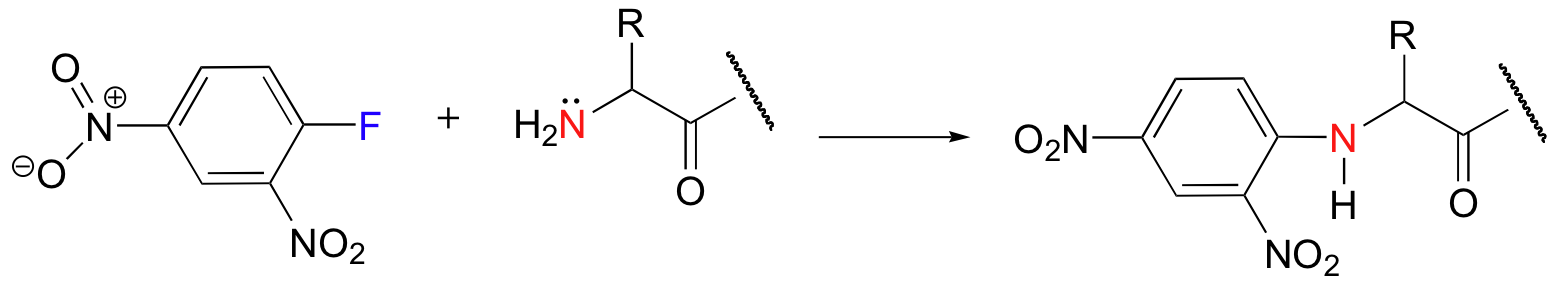
nucleophilic aromatic substitution
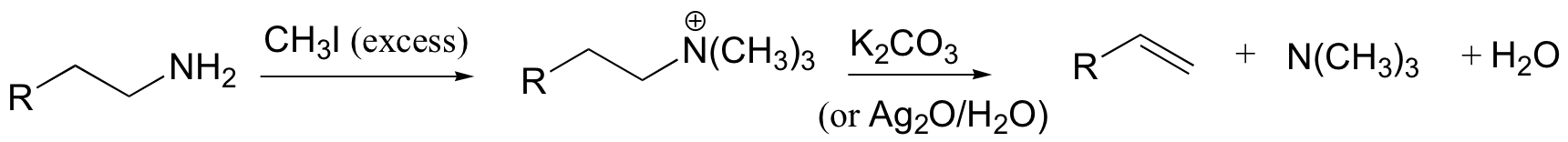
Hoffman elimination - least substituted alkene produced

Cope elimination
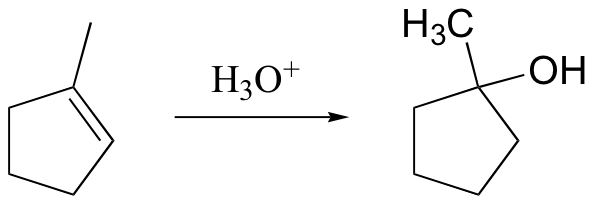
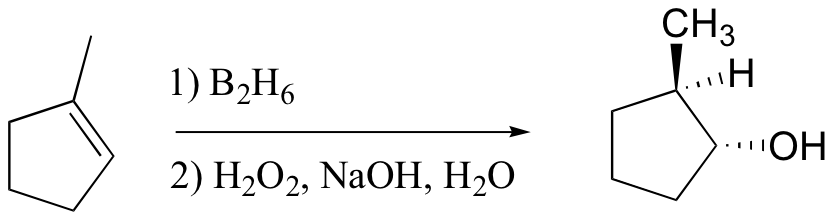
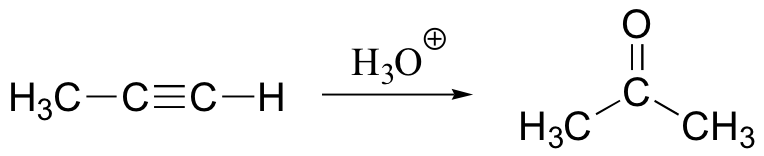
anti-Markovnikov addition of water to alkene. Notice syn addition!
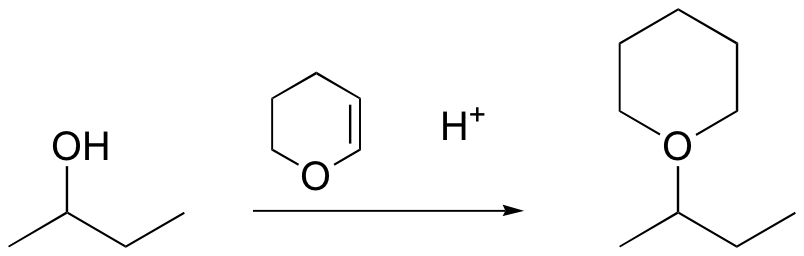
method to protect alcohol – remove with H3O+
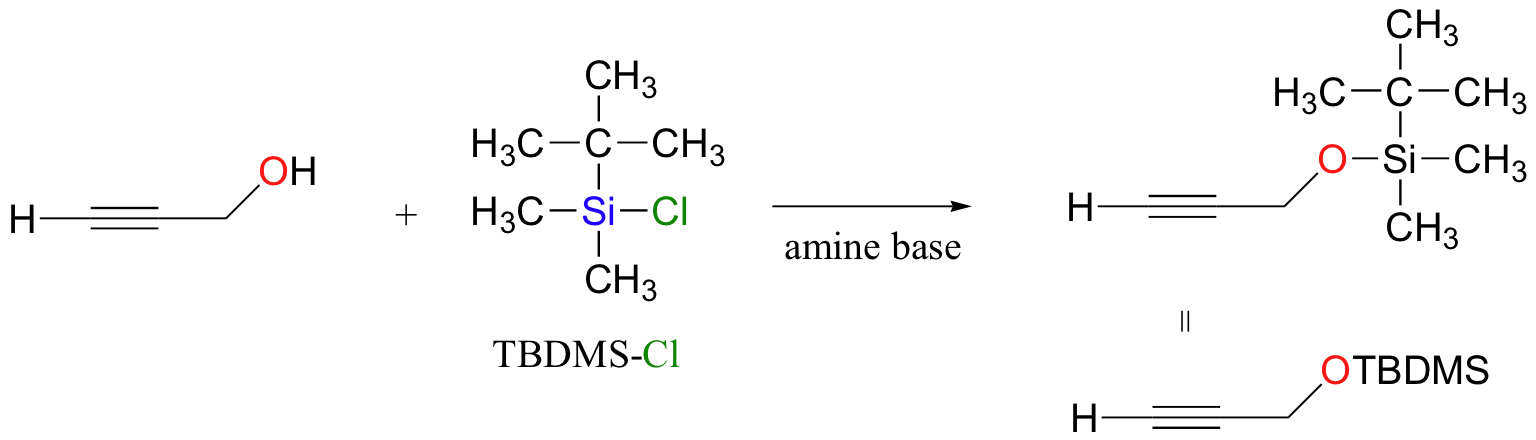
another alcohol protecting group: remove with F- ion
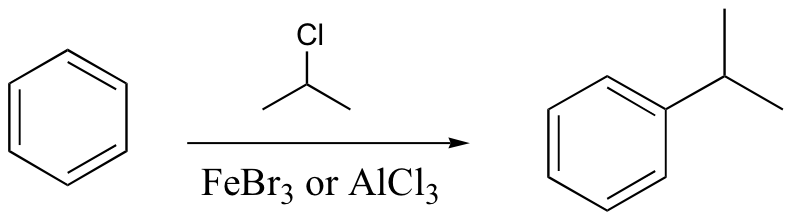
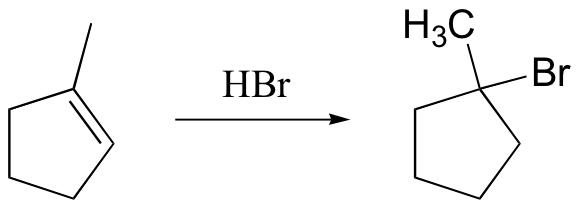
watch out for the possibility of carbocation rearrangements!
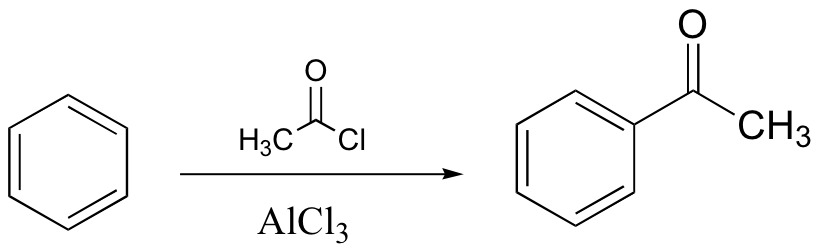
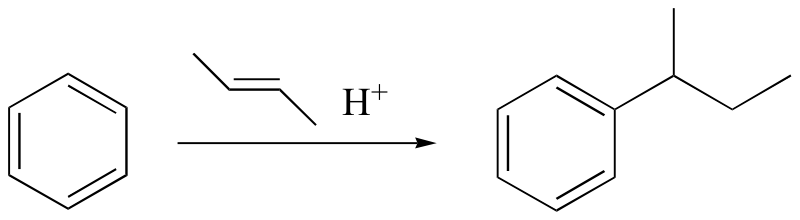
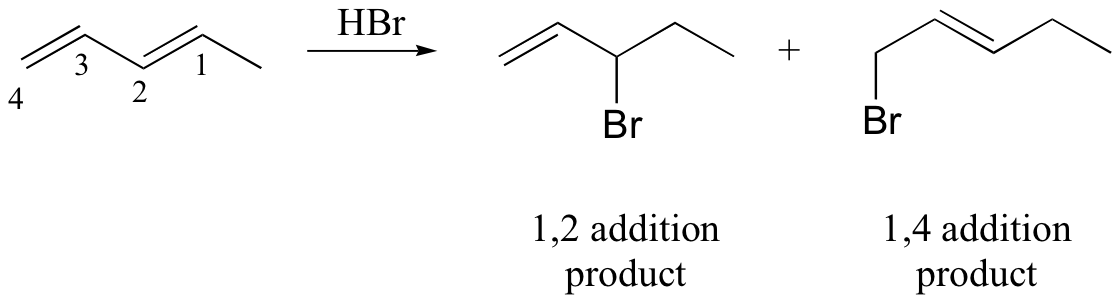
watch out for the possibility of carbocation rearrangements!
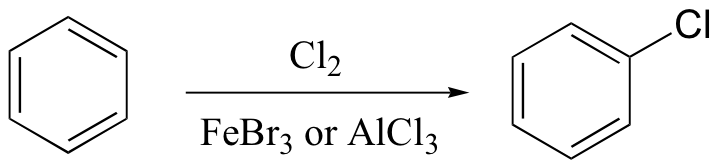
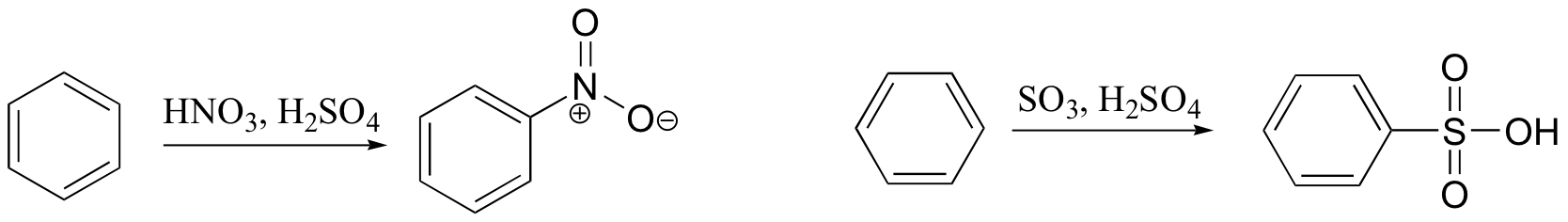
ortho-para directing vs. meta-directing groups
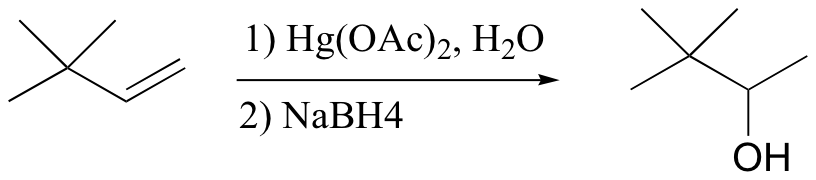
Markovnikov addition of water without possibility of carbocation shifting
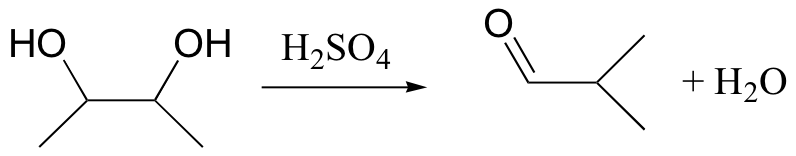
pinacol rearrangement
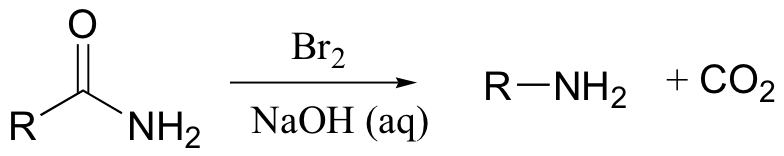
Hoffman rearrangement
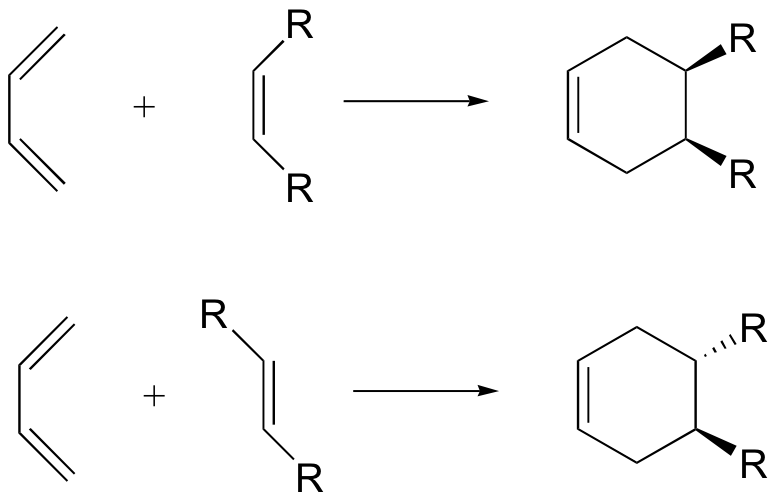
Diels-Alder: cis/trans stereoselectivity
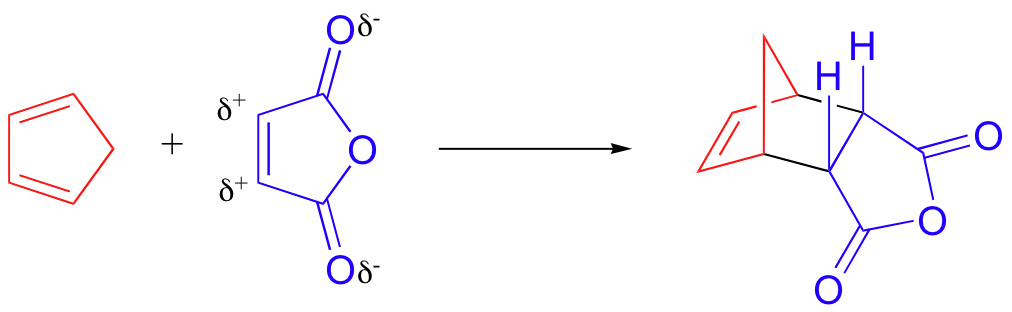
bicyclic Diels-Alder product - no stereoselectivity
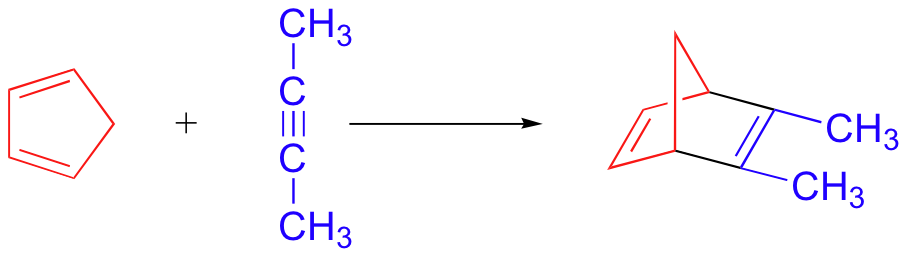
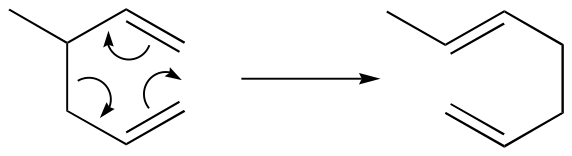
Cope rearrangement
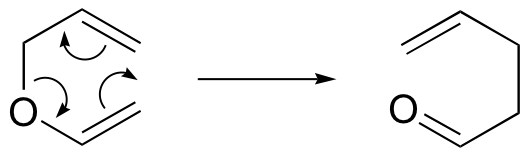
Claisen rearrangement
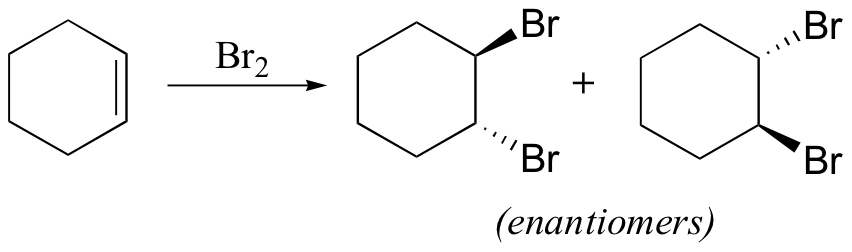
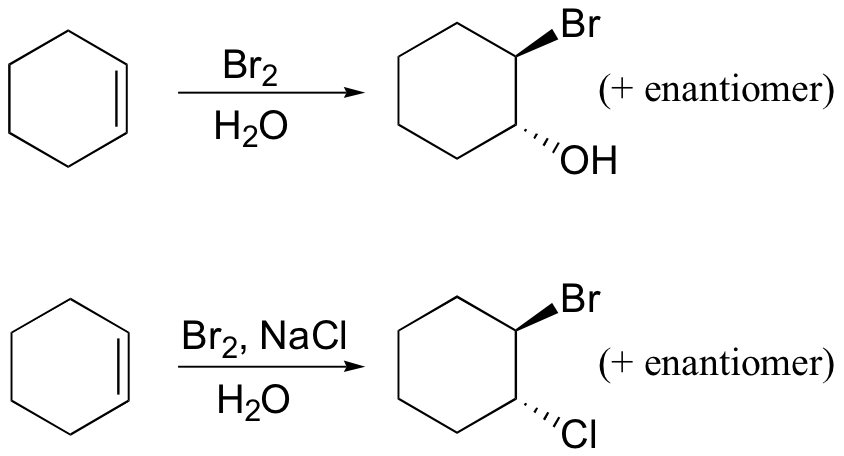
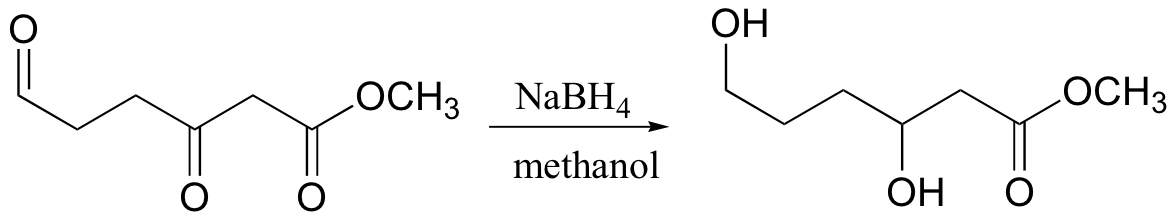
reduces aldehydes/ketones, but not carboxylic acid derivatives
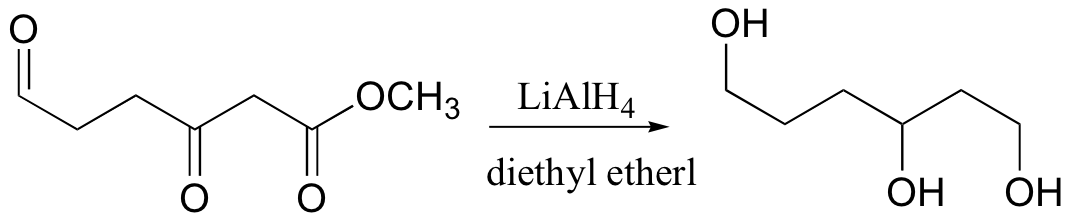
reduces aldehydes, ketones, carboxylic acid derivatives
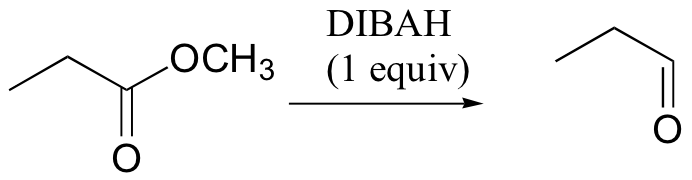
can reduce ester/amide to aldehyde (LiAlH4 can't do this)
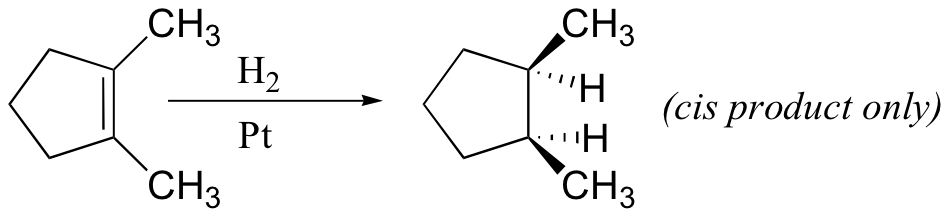
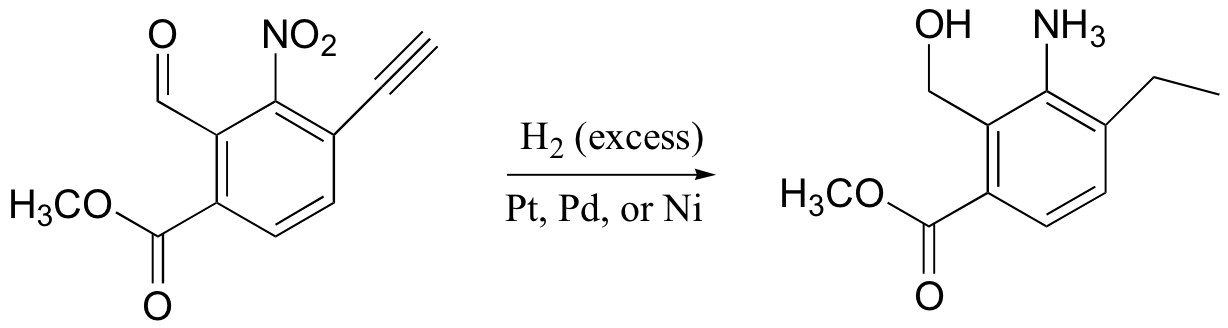
alkynes, aldehyde, ketones, nitro groups also reduced by H2/Pt (but not acid derivatives!)
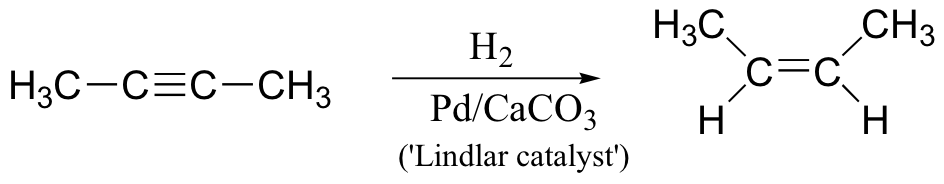
alkyne to cis-alkene
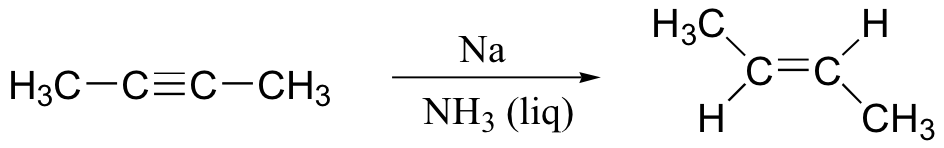
alkyne to trans-alkene
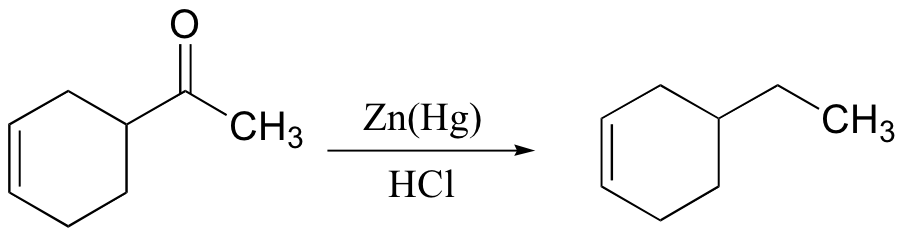
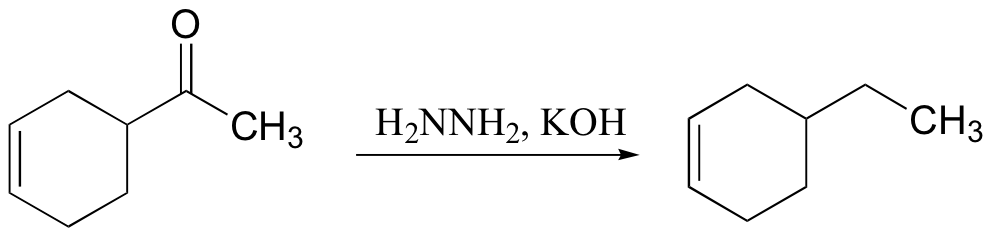
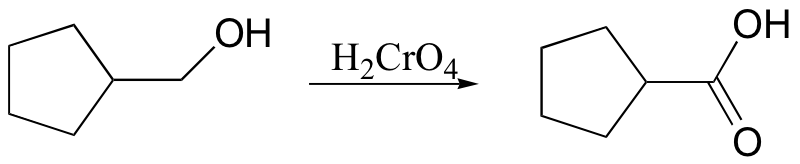
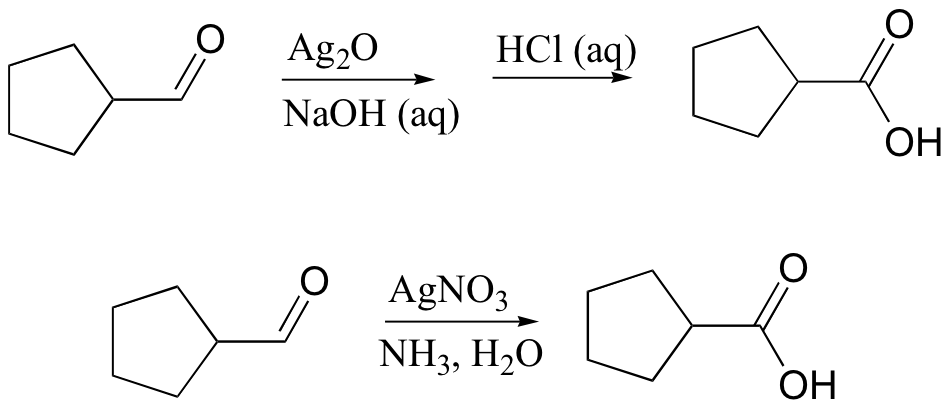
primary alcohol to acid
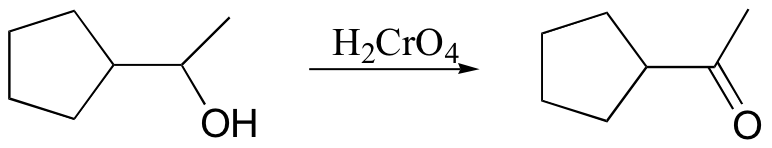
secondary alcohol to ketone
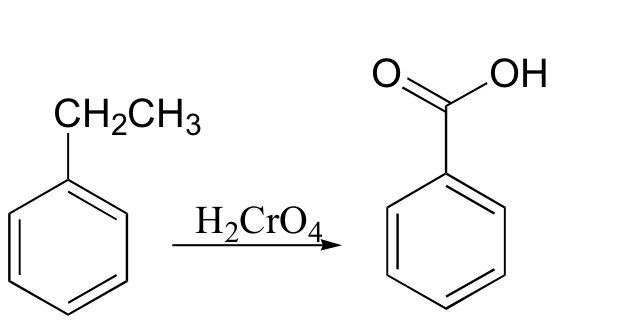
oxidation at the benzylic position
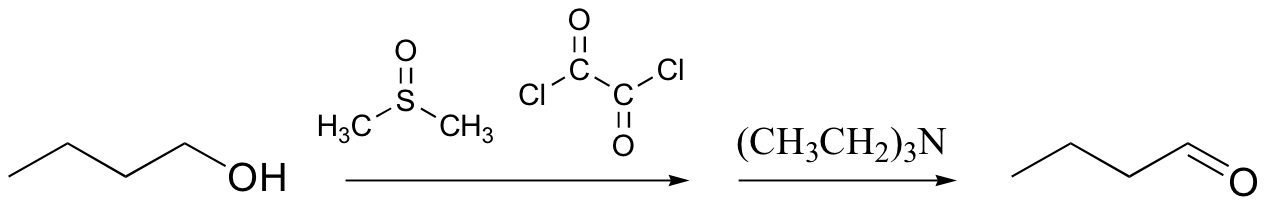
Swern oxidation
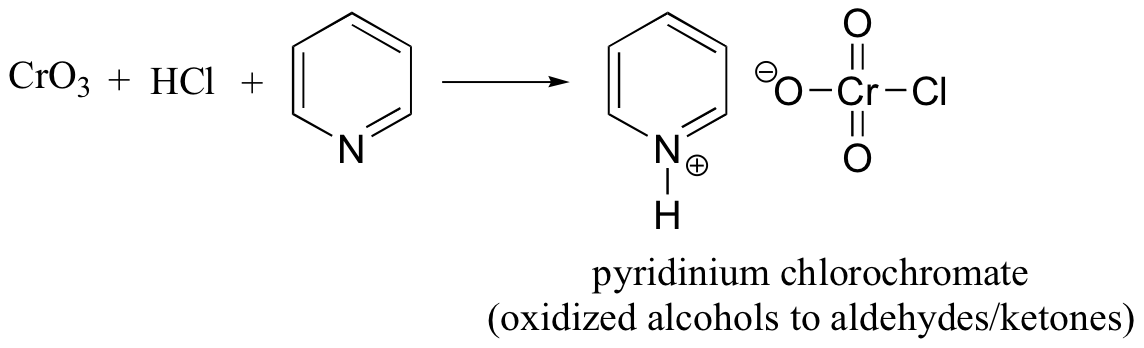
abbreviated PCC
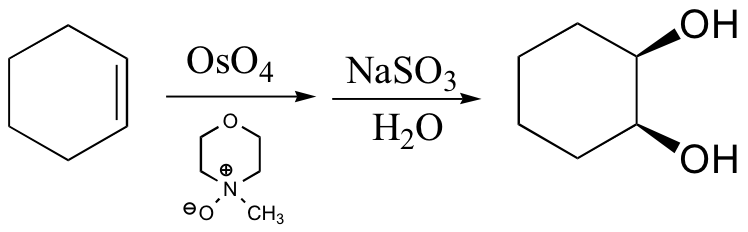
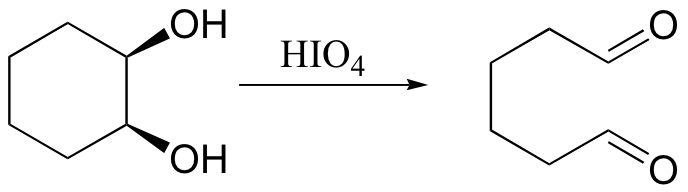
cis diols cleaved, not trans
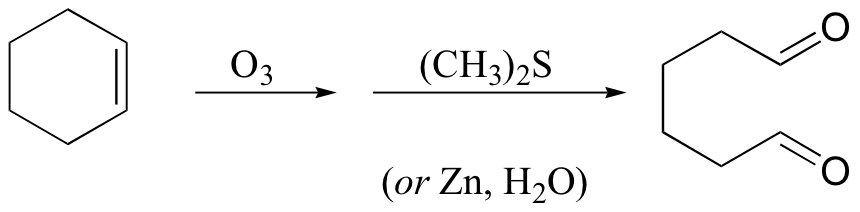
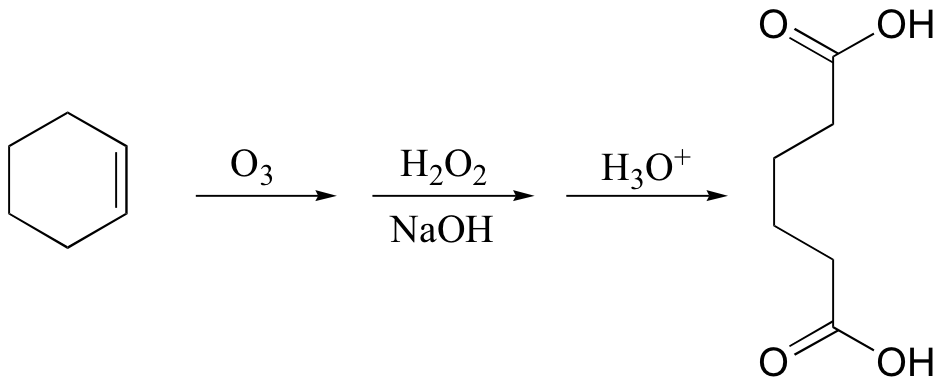
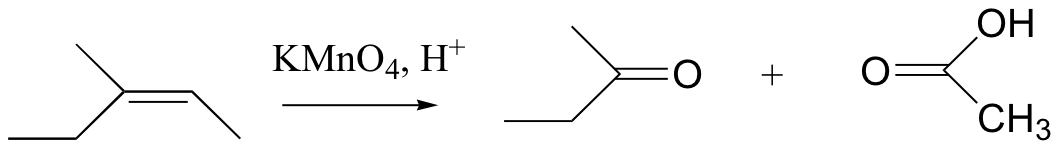
KMnO4 also oxidizes primary alcohols and aldehydes to acids
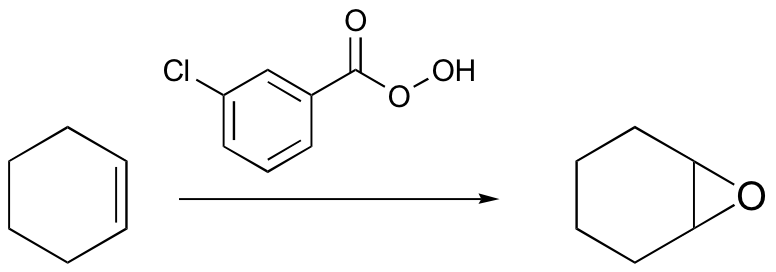
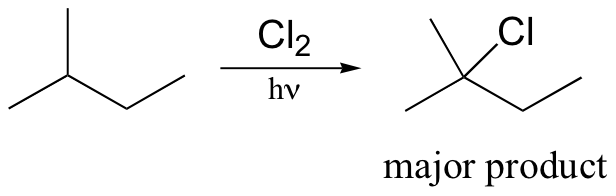
radical halogenation is regiospecific – depends on stablity of radical intermediate
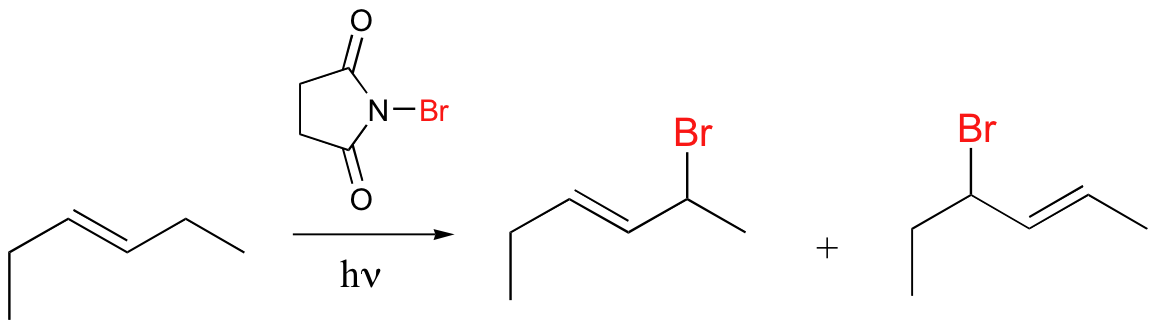
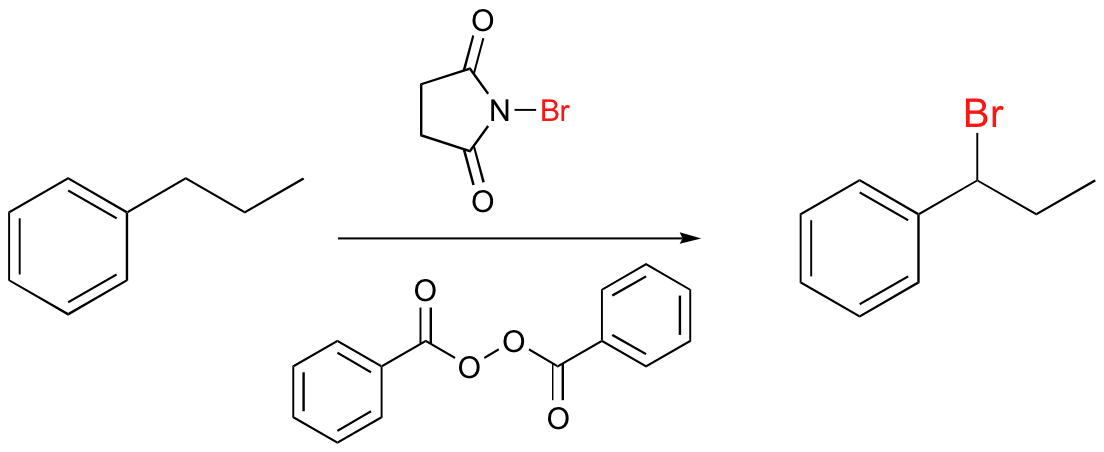
NBS can be source of Br in radical halogenation reactions
regiospecificity: benzylic / allylic


