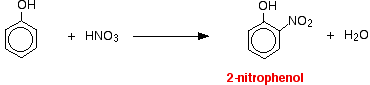Ring Reactions of Phenol
- Page ID
- 3988
This page looks at the way the reactions of the benzene ring in phenol are modified by the presence of the attached -OH group. It covers the reactions of phenol with bromine water and with nitric acid.
Activation of the ring
The -OH group attached to the benzene ring in phenol has the effect of making the ring much more reactive than it would otherwise be. For example, as you will find below, phenol will react with a solution of bromine in water (bromine water) in the cold and in the absence of any catalyst. It also reacts with dilute nitric acid, whereas benzene itself needs a nitrating mixture of concentrated nitric acid and concentrated sulfuric acid.
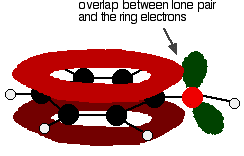
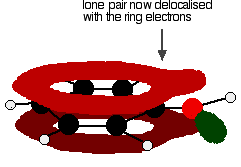
Figure: One of the lone pairs on the oxygen atom in the -OH group overlaps with the delocalised ring electron system giving a structure rather like the right.
The donation of the oxygen's lone pair into the ring system increases the electron density around the ring. A benzene ring undergoes substitution reactions in which the ring electrons are attacked by positive ions or the slightly positive parts of molecules. In other words, it undergoes electrophilic substitution. If you increase the electron density around the ring, it becomes even more attractive to incoming electrophiles. That's what happens in phenol.
The directing effect of the -OH group
The -OH group has more activating effect on some positions around the ring than others (for reasons which go beyond UK A level). That means that incoming groups will go into some positions much faster than they will into others.
The net effect of this is that the -OH group has a 2,4-directing effect. That means that incoming groups will tend to go into the 2- position (next door to the -OH group) or the 4- position (opposite the -OH group). You will get hardly any of the 3- isomer formed - it is produced too slowly.
| Example 1: Reaction with Bromine Water |
|---|
|
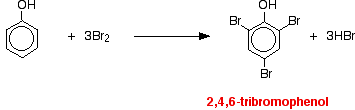
If bromine water is added to a solution of phenol in water, the bromine water is decolorized and a white precipitate is formed which smells of antiseptic. The precipitate is 2,4,6-tribromophenol.
Notice the multiple substitution around the ring - into all the activated positions. (The 6- position is, of course, just the same as the 2- position. Both are next door to the -OH group.) |
| Example 2: Reactions with Nitric Acid |
|---|
|
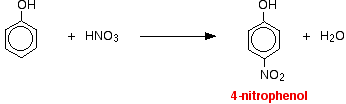
The reactions with nitric acid are complicated because nitric acid is an oxidizing agent, and phenol is very easily oxidized to give complex tarry products. What follows misses all that complication out, and just concentrates on the ring substitution which happens as well. With dilute nitric acid: Phenol reacts with dilute nitric acid at room temperature to give a mixture of 2-nitrophenol and 4-nitrophenol.
With concentrated nitric acid: With concentrated nitric acid, more nitro groups substitute around the ring to give 2,4,6-trinitrophenol (common name: picric acid).
|
Contributors
Jim Clark (Chemguide.co.uk)