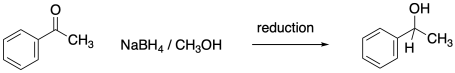CO17. Oxidation
- Page ID
- 4225
CO17. Organic Oxidation
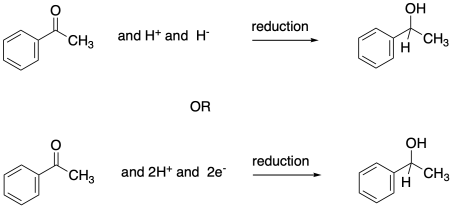
You may recall that conversion of an aldehyde or ketone to an alcohol is referred to as a reduction. The hydride from an NADH molecule or a BH4- anion acts as a nucleophile, adding H- to the carbonyl carbon. A proton source can then protonate the oxygen of the resulting alkoxide ion, forming an alcohol.
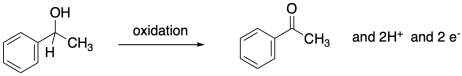
In this reduction, two electrons and two protons are donated to the carbonyl compound to produce an alcohol.
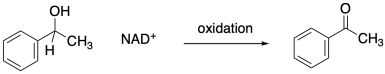
The opposite process, the loss of two protons and two electrons from an alcohol to form a ketone or aldehyde, is an oxidation.
In biological pathways, oxidation is often the microscopic reverse of reduction. That means that the products of a reduction, NAD+ and an alcohol, could react together under the right circumstances to form NADH and a carbonyl. The reduction of NAD+ by a hydride donor is possible because, although the NAD+ loses the aromaticity of its nicotinamide ring upon becoming NADH, it also loses its positive charge. Charge stabilization is frequently an energetic problem for molecules.
Notice that this same argument has been used to look at biological oxidation and reduction in both directions. That's because each side of the equation has some energetic advantage. The NAD+ is aromatic. The NADH is neutral. This is a well-balanced system energetically, and the balance of the reaction can be tipped in either direction. The direction of the reaction is influenced by the surroundings.
In biology, NADH and NAD+ are just cofactors in a reaction. A reduction would take place in an enzyme that specifically carries out reductions, and an oxidation would occur in an enzyme specific for the oxidation. The enzyme is just a large protein that holds the substrate (such as the alcohol) and cofactor (such as NAD+) together in close proximity. Acidic and basic sites are provided by nearby amino acid residues, and other amino acid side chains may push the reactants into optimum position for one reaction or another.
In the laboratory, enzymes and cofactors can sometimes be added to reaction flasks in order to oxidize or reduce substrates. Sometimes these reactions are not convenient, however. In addition, there are a number of other ways to carry out oxidations and reductions. For example, addition of a hydride could be accomplished via addition of sodium borohydride. That would result in reduction of a carbonyl to an alcohol.
By analogy to the NADH / NAD+ approach in nature, the easiest way to oxidize an alcohol to a carbonyl would be to remove a hydride and a proton. Nature uses enzymes to bring reactants together for transformations like this. In the laboratory, metals are often used to bring two reactants together. This is sometimes true in enzymes, too: a metal at the enzyme active site may tether two molecules together, or even activate one so that it is ready to react. Metals can "hold onto" reactants because molecules with lone pairs will often coordinate to metals; a lone pair is shared with the electrophilic metal.
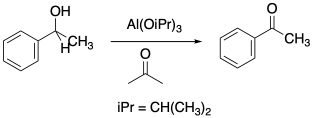
In a process known as an Oppenauer oxidation, a Lewis acidic metal such as aluminum is used for this tethering role. Aluminum tris(isopropoxide), Al(OCH(CH3)2)3 carries out the oxidation of an alcohol when dissolved in acetone or propanone, (CH3)2C=O. In this reaction, the acetone is a sacrificial oxidant. When both the alcohol and a molecule of acetone are coordinated to the aluminum, a hydride is transferred from the alcohol carbon to the carbonyl carbon of the coordinated acetone. The alcohol is converted to an aldehyde or ketone and the acetone is converted to isopropanol.
This general type of reaction, hydride transfer reduction, has been adapted by Ryoji Noyori, of Nagoya University in Japan, to produce a single enantiomer of a chiral alcohol product. Noyori's work on this reaction, and others, led to him being awarded the Nobel Prize in Chemistry in 2001.
Problem CO17.1.
Show the two enantiomers that could be produced from reduction of acetophenone, CH3(CO)C6H5.
Problem CO17.2.
Provide a mechanism with arrows for the Oppenauer oxidation of benzyl alcohol, C6H5CH2OH.
Problem CO17.3.
Explain why acetone is used as the solvent in an Oppenauer oxidation.
Problem CO17.4.
Meerwein-Ponndorf-Verley reduction of a ketone is carried out with aluminum tris(isopropoxide) in isopropanol as solvent. Provide a mechanism for the reduction of acetophenone, CH3(CO)C6H5, via this reaction.
Problem CO17.5.
Explain why isopropanol is used as the solvent in a Meerwein-Pondorf-Verley reduction.
A second general method for alcohol oxidation employs a "redox-active" transition metal to accept a pair of electrons from from an alcohol during the oxidation. Because oxidation of an alcohol formally involves the loss of two electrons and two protons, a proton acceptor is also involved in this oxidation. There are many redox-active metals, but one of the most commonly used is Cr(VI). When Cr(VI) accepts a pair of electrons, it becomes Cr(IV).
In order to look at how chromium oxidation works, we'll use chromium oxide, CrO3, as an oxidant and water as a solvent. Note that water could also act as a proton acceptor or proton shuttle, moving protons from one place to another as needed. To carry out an oxidation, a number of events need to happen.
- The alcohol needs to bind to the chromium.
- A proton needs to be removed. This event is helped by the formal positive charge on the alcohol after it donates a lone pair to the chromium.
- A second proton must be removed and a pair of electrons given to the chromium for good.
In reality, CrO3 isn't used that often as an oxidant. It tends to catch fire when mixed with organic compounds. Instead, a variety of other chromium compounds are used.
Problem CO17.6.
In determining an oxidation state, we imagine giving both electrons in a bond to the more electronegative atom and looking at the resulting charges on the ions that result. Assuming all of the oxygens in chromium oxide can be thought of as dianions, confirm that the chromium can be thought of as a Cr6+ cation (in other words, in oxidation state Cr(VI)).
Problem CO17.7.
By the reasoning used in the previous question, determine the oxidation state of the transition metal in the following compounds. Note that in some cases, there is an anion and cation in the compound.
a) KMnO4 b) NaIO4 c) Ag2O d) OsO4 d) (CH3CH2CH2)4N RuO4