CO5. Nucleophilic Addition
- Page ID
- 4235
CO5. Introduction to Carbonyl Addition
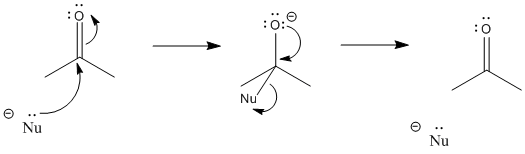
Carbonyls act most importantly as electrophiles. They attract a pair of electrons from a nucleophile. When that happens, a bond forms between the nucleophile and the carbonyl carbon.
At the same time, the carbon-oxygen bond breaks. We think of that as a consequence of donating a pair of electrons into the LUMO of the carbonyl. The LUMO on the carbonyl is the C-O pi antibonding orbital. When that orbital is populated, there is no longer a net lowering in energy due to the pi interaction between the carbon and oxygen. The pi bond breaks. The electron pair from the pi bond goes to the oxygen, the more electronegative of the two atoms in the original bond. It becomes a lone pair.
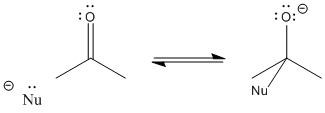
After the pi bond breaks, the reaction reaches a branching point or decision point. The reaction may go forwards or backwards.
In other words, this reaction can occur in equilibrium.
To go backwards, the reaction simply slides into reverse. A lone pair on the oxygen donates to the carbon, forming a pi bond again, and pushes the nucleophile off. Whether the reaction ends up going forward or sliding backward depends partly on the relative stability of those two ends of the reaction. That’s often very difficult to assess qualitatively, because there are too many factors involved. However, one factor that plays a role is charge stability. Because an “O minus” or alkoxide is produced in this reaction, if the original nucleophile was a more reactive ion than an alkoxide, the reaction probably goes to the right. If the nucleophile was less reactive than alkoxide, the reaction can easily go to the left again.
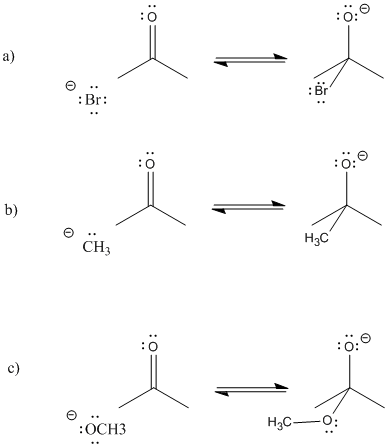
Problem CO5.1.
Provide curved arrows and predict the direction of equilibrium in the following cases.
What happens after the initial equilibrium? In most cases, the alkoxide that is formed will become protonated. It will pick up a proton to become an alcohol. The source of the proton may be an acid, deliberately added to provide the H+. Alternatively, it may just be a very slightly acidic molecule such as water or another alcohol.