D. The Dehydration of Propan-2-ol
- Page ID
- 3786
This page looks at the mechanism for the acid catalysed dehydration of propan-2-ol.
The dehydration of propan-2-ol is taken as an simple example of the way that secondary and tertiary alcohols dehydrate.
Primary alcohols like ethanol use a different mechanism, and ethanol is discussed separately on another page. You will find a link to this from the elimination mechanisms menu.
You will also find a link there to a page on the dehydration of more complicated alcohols where more than one product may be formed.
The dehydration of propan-2-ol
The facts
Propan-2-ol can be dehydrated to give propene by heating it with an excess of concentrated sulphuric acid at about 170°C.
Concentrated phosphoric(V) acid, H3PO4, can be used instead.
![]()
The acids aren't written into the equation because they serve as catalysts. If you like, you could write, for example, "conc H2SO4" over the top of the arrow.
Notice that the -OH group is lost, together with a hydrogen from a next-door carbon - it doesn't matter which one. If you chose the other one, you would get CH3CH=CH2. That's the same molecule flipped over.
The mechanism - the full version
We are going to discuss the mechanism using sulphuric acid. Afterwards, we'll describe how you can use a simplified version which will work for any acid, including phosphoric(V) acid.
In the first stage, one of the lone pairs of electrons on the oxygen picks up a hydrogen ion from the sulphuric acid. The alcohol is said to be protonated.
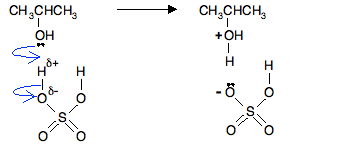
The negative ion produced is the hydrogensulphate ion, HSO4-.
Notice that the oxygen atom in the alcohol has gained a positive charge. That charge has to be there for two reasons:
- On the left hand side of the equation you start with two overall neutral molecules. Assuming you forgot about the positive charge, you would end up with a neutral species and a negative ion on the right. Charges must balance in equations, so something is wrong.
- The oxygen looks wrong! The oxygen atom is joined to 3 things rather than its usual 2. Oxygen can only join to 3 things if it carries a positive charge.
In the second stage of the reaction the protonated propan-2-ol loses a water molecule to leave a carbocation (previously known as a carbonium ion) - an ion with a positive charge on a carbon atom.
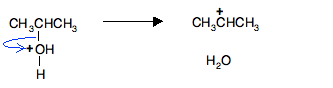
The carbon atom is positive because it has lost the electron that it originally contributed to the carbon-oxygen bond. Both of the electrons in that bond have moved onto the oxygen atom, neutralising the oxygen's charge.
Finally, a hydrogensulphate ion (from the sulphuric acid) pulls off a hydrogen ion from the carbocation, and a double bond forms.
The mechanism - a simplified version
People normally quote a simplified version of this mechanism. Instead of showing the full structure of the sulphuric acid, you write it as if it were simply a hydrogen ion, H+. That leaves the full mechanism:
An advantage of this (apart from the fact that it doesn't require you to draw the structure of sulphuric acid) is that it can be used for any acid catalyst without changing it at all. For example, if you use this version, you wouldn't need to worry about the structure of phosphoric(V) acid.


