Pushing Arrows
- Page ID
- 1142
In organic chemistry, it is important to understand the concept of electron flow. In polar reaction mechanisms, such as the nucleophilic substitution reactions of haloalkanes, electron flow will be designated by arrows indicating the movement of electrons from electron rich regions to electron poor regions.
Introduction
In considering this concept, we must look at the two types of arrows provided in the mechanisms shown below. The curved arrows indicate the movement of electrons. The first type of arrow, shown in pink, originates from the electron pair of the nucleophile and extends to the electrophilic carbon of the haloalkane. This type of movement does not indicate that electrons leave the nucleophile; rather, it means that electrons become shared between the nucleophile and the electrophilic atom.
The second type of curved arrow, also shown in pink, originates from the R-X bond and extends to the halogen. This indicates cleavage of the bond, whereby the electron pair becomes separated from R, the electrophilic carbon, and ends up on the halogen atom.
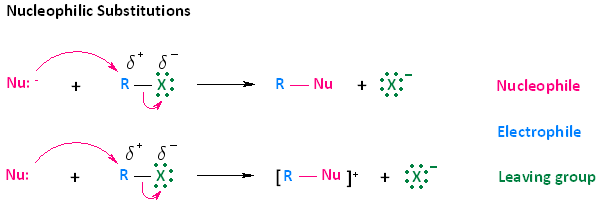
While we are using the concept of nucleophilic substitution mechanisms to explain electron flow, it is very important to understand that this concept will be applied in nearly all the mechanisms you learn throughout your course of study. The simplest way to think about this in any mechanism you learn is that electrons will be pushed from an electron rich species or site to an electron poor species or site, and the direction of the curved arrow will indicate this.
Contributors
- Rachael Curtis

