SN2
- Page ID
- 1146
Nucleophilic Substitution
Previously (Physical Properties of Haloalkanes), we learned that haloalkanes contain a polarized C-X bond, leaving a carbon that is partially positive and a halogen that is partially negative.
- An electrophile is an electron poor species that can accept a pair of electrons. A carbon that is connected to a halogen in a haloalkane, for example, is an electrophilic carbon.
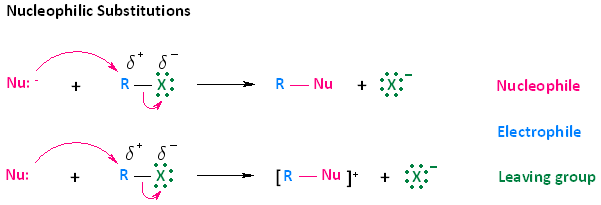
- A nucleophile is an electron rich species that can donate a pair of electrons. You will be exposed to many different kinds of nucleophiles throughout your course of study. Some nucleophiles will be negatively charged species; others will be neutral.
- Nucleophiles can react with electrophiles. One way in which this occurs is through a process called nucleophilic substitution. In nucleophilic substitution reactions, an electron rich nucleophile bonds with or attacks an electron poor electrophile, resulting in the displacement of a group or atom called the leaving group.
- Nucleophilic substitution of haloalkanes can be described by two reactions. These two types of reactions are shown in the diagram below. In the first reaction, a negatively charged nucleophile attacks the electrophilic carbon of a haloalkane. Upon attack, the leaving group, which is the halogen of the haloalkane, leaves. The end result is a neutral R-Nu species and an anion. In the second reaction, a neutral nucleophile attacks the electrophilic carbon of a haloalkane. The end result of this attack however, is positively charged product and an anion.
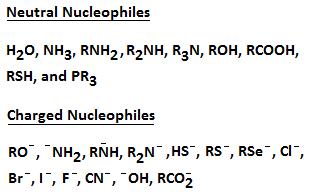
Examples of Negatively Charged and Neutral Nucleophiles
In the SN2 reaction, the addition of the nucleophile and the departure of the leaving group occur in a concerted(taking place in a single step) manner, hence the name SN2: substitution, nucleophilic, bimolecular. In the SN2 reaction, the nucleophile approaches the carbon atom to which the leaving group is attached. As the nucleophile forms a bond with this carbon atom, the bond between the carbon atom and the leaving group breaks. The bond making and bond breaking actions occur simultaneously. Eventually, the nucleophile has formed a complete bond to the carbon atom and the bond between the carbon atom and the leaving group is completely broken.
In the image below, we introduce the concepts of arrow pushing and of a reaction mechanism. Recall that electrons compose the bonds in molecules. Hence for any reaction to occur, electrons must move. In arrow pushing, the movement of electrons is indicated by arrows. Arrows may show electrons forming or breaking bonds or traveling as lone pairs or negative charges on atoms. A complete schematic showing all steps in a reaction, including arrow pushing to indicate the movement of electrons, constitutes a reaction mechanism. In the reaction mechanism below, observe that the electrons from a negative charge on the nucleophile "attack" or form a bond with the carbon atom to which the leaving group is attached. In the center image, the partially formed bond is visible, as well as the partially broken bond to the leaving group. In the final image, the bond to the leaving group is broken when its electrons become a negative charge on the leaving group.
Figure 1: SN2 reaction showing concerted, bimolecular participation of nucleophile and leaving group
A consequence of the concerted, bimolecular nature of the SN2 reaction is that the nucleophile must attack from the side of the molecule opposite to the leaving group. This geometry of reaction is called back side attack. In a back side attack, as the nucleophile approaches the molecule from the side opposite to the leaving group, the other three bonds move away from the nucleophile and its attacking electrons. Eventually, these three bonds are all in the same plane as the carbon atom (center image). As the bond to the leaving group breaks, these bonds retreat farther away from the nucleophile and its newly formed bond to carbon atom. As a result of these geometric changes, the stereochemical configuration of the molecule is inverted during an SN2 reaction to the opposite enantiomer. This stereochemical change is called inversion of configuration.
The concerted mechanism and nature of the nucleophilic attack in an SN2 reaction give rise to several important results:
- The rate of the reaction depends on the concentration of both the nucleophile and the molecule undergoing attack. The reaction requires a collision between the nucleophile and the molecule, so increasing the concentration of either will increase the rate of the reaction.
- Since the unique geometry of back side attack is required, the most important factor in determining whether an SN2 reaction will occur is steric effects. Steric effects refer to the unfavorable interaction created when atoms are brought too close together. In effect, if the nucleophile or the molecule undergoing attack have too many substituents or substituents which are too bulky, the reaction cannot occur since the nucleophile will be unable to get close enough to the molecule to do a backside attack.
Now let's look at two actual examples of these two general equations. In the first reaction shown below, the negative nucleophile, hydroxide, reacts with methyl iodide. Hydroxide takes the place of the leaving group, iodide, forming neutral methanol and an iodide ion. This reaction is the same as the first type of nucleophilic substitution shown above. In the second reaction shown below, the nuetral nucleophile, ammonia, reacts with iodoethane. Ammonia takes the place of the leaving group, iodide, forming the positively charged product, ethylammonium iodide, and an iodide ion. This reaction is the same as the second type of nucleophilic substitution shown above.
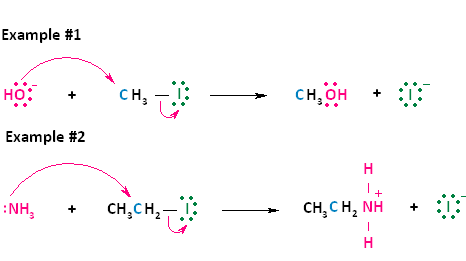
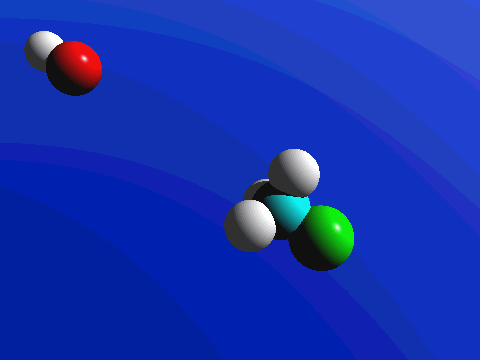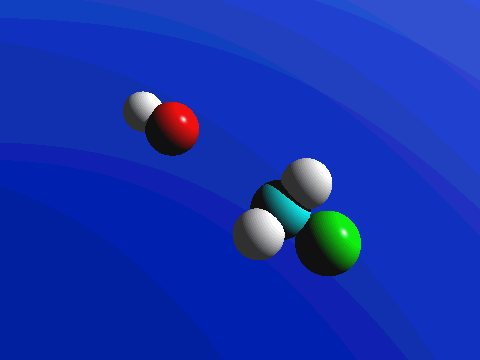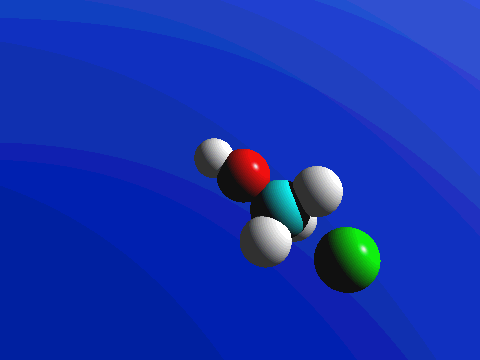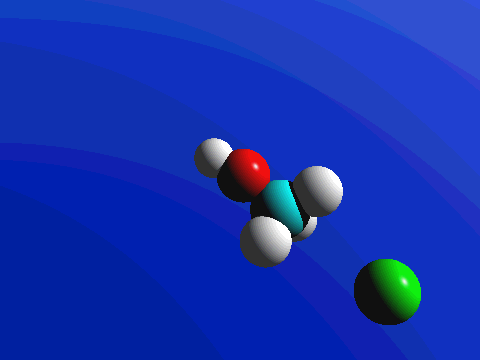

Figure 2. SN2 reaction of methyl chloride and hydroxide ion (left) and ammonia reaction with HCl (right).
Four Factors to Consider in Determining the Relative Ease at Which SN2 Displacement Occurs
- The nature of the leaving group (SN2 Reactions-The Leaving Group)
- The reactivity of the nucleophile (SN2 Reactions-The Nucleophile)
- The solvent (SN2 Reactions-The Nucleophile)
- The structure of the alkyl portion of the substrate (SN2 Reactions-The Substrate)
Next section: Using Electron-Pushing Arrows
Contributors
- Rachael Curtis (UCD)
- Jonathan Mooney (McGill University)

