Lewis Concept of Acids and Bases
- Page ID
- 1282
Acids and bases are an important part of chemistry. One of the most applicable theories is the Lewis acid/base motif that extends the definition of an acid and base beyond H+ and OH- ions as described by Brønsted-Lowry acids and bases.
The Brønsted acid-base theory has been used throughout the history of acid and base chemistry. However, this theory is very restrictive and focuses primarily on acids and bases acting as proton donors and acceptors. Sometimes conditions arise where the theory does not necessarily fit, such as in solids and gases. In 1923, G.N. Lewis from UC Berkeley proposed an alternate theory to describe acids and bases. His theory gave a generalized explanation of acids and bases based on structure and bonding. Through the use of the Lewis definition of acids and bases, chemists are now able to predict a wider variety of acid-base reactions. Lewis' theory used electrons instead of proton transfer and specifically stated that an acid is a species that accepts an electron pair while a base donates an electron pair.
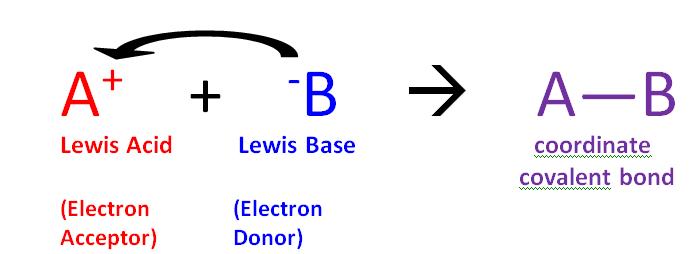
The reaction of a Lewis acid and a Lewis base will produce a coordinate covalent bond (Figure \(\PageIndex{1}\)). A coordinate covalent bond is just a type of covalent bond in which one reactant gives it electron pair to another reactant. In this case the lewis base donates its electrons to the Lewis acid. When they do react this way the resulting product is called an addition compound, or more commonly an adduct.
- Lewis Acid: a species that accepts an electron pair (i.e., an electrophile) and will have vacant orbitals
- Lewis Base: a species that donates an electron pair (i.e., a nucleophile) and will have lone-pair electrons
Lewis Acids
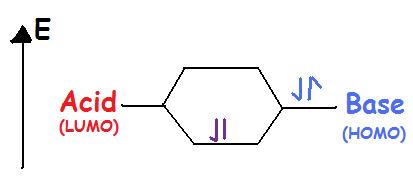
Lewis acids accept an electron pair. Lewis Acids are Electrophilic meaning that they are electron attracting. When bonding with a base the acid uses its lowest unoccupied molecular orbital or LUMO (Figure 2).
- Various species can act as Lewis acids. All cations are Lewis acids since they are able to accept electrons. (e.g., Cu2+, Fe2+, Fe3+)
- An atom, ion, or molecule with an incomplete octet of electrons can act as an Lewis acid (e.g., BF3, AlF3).
- Molecules where the central atom can have more than 8 valence shell electrons can be electron acceptors, and thus are classified as Lewis acids (e.g., SiBr4, SiF4).
- Molecules that have multiple bonds between two atoms of different electronegativities (e.g., CO2, SO2)
Lewis Bases
Lewis Bases donate an electron pair. Lewis Bases are Nucleophilic meaning that they “attack” a positive charge with their lone pair. They utilize the highest occupied molecular orbital or HOMO (Figure 2). An atom, ion, or molecule with a lone-pair of electrons can thus be a Lewis base. Each of the following anions can "give up" their electrons to an acid, e.g., \(OH^-\), \(CN^-\), \(CH_3COO^-\), \(:NH_3\), \(H_2O:\), \(CO:\). Lewis base's HOMO (highest occupied molecular orbital) interacts with the Lewis acid's LUMO (lowest unoccupied molecular orbital) to create bonded molecular orbitals. Both Lewis Acids and Bases contain HOMO and LUMOs but only the HOMO is considered for Bases and only the LUMO is considered for Acids (Figure \(\PageIndex{2}\)).
Complex Ion / Coordination Compounds
Complex ions are polyatomic ions, which are formed from a central metal ion that has other smaller ions joined around it. While Brønsted theory can't explain this reaction Lewis acid-base theory can help. A Lewis Base is often the ligand of a coordination compound with the metal acting as the Lewis Acid (see Oxidation States of Transition Metals).
\[Al^{3+} + 6 H_2O \rightleftharpoons [Al(H_2O)_6]^{3+} \label{1}\]
The aluminum ion is the metal and is a cation with an unfilled valence shell, and it is a Lewis Acid. Water has lone-pair electrons and is an anion, thus it is a Lewis Base.
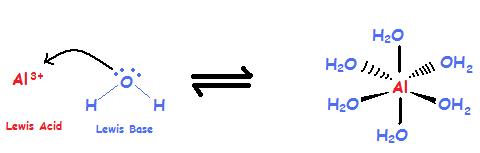
The Lewis Acid accepts the electrons from the Lewis Base which donates the electrons. Another case where Lewis acid-base theory can explain the resulting compound is the reaction of ammonia with Zn2+.
\[ Zn^{2+} + 4NH_3 \rightarrow [Zn(NH_3)_4]^{4+} \label{2}\]
Similarly, the Lewis Acid is the zinc Ion and the Lewis Base is NH3. Note how Brønsted Theory of Acids and Bases will not be able to explain how this reaction occurs because there are no \(H^+\) or \(OH^-\) ions involved. Thus, Lewis Acid and Base Theory allows us to explain the formation of other species and complex ions which do not ordinarily contain hydronium or hydroxide ions. One is able to expand the definition of an acid and a base via the Lewis Acid and Base Theory. The lack of \(H^+\) or \(OH^-\) ions in many complex ions can make it harder to identify which species is an acid and which is a base. Therefore, by defining a species that donates an electron pair and a species that accepts an electron pair, the definition of a acid and base is expanded.
Amphoterism
As of now you should know that acids and bases are distinguished as two separate things however some substances can be both an acid and a base. You may have noticed this with water, which can act as both an acid or a base. This ability of water to do this makes it an amphoteric molecule. Water can act as an acid by donating its proton to the base and thus becoming its conjugate acid, OH-. However, water can also act as a base by accepting a proton from an acid to become its conjugate base, H3O+.
- Water acting as an Acid:
\[H_2O + NH_3 \rightarrow NH_4^+ + OH^- \label{3}\]
- Water acting as a Base:
\[H_2O + HCl \rightarrow Cl^- + H_3O^+ \label{4}\]
You may have noticed that the degree to which a molecule acts depends on the medium in which the molecule has been placed in. Water does not act as an acid in an acid medium and does not act as a base in a basic medium. Thus, the medium which a molecule is placed in has an effect on the properties of that molecule. Other molecules can also act as either an acid or a base. For example,
\[Al(OH)_3 + 3H^+ \rightarrow Al^{3+} + 3H_2O \label{5}\]
- where Al(OH)3 is acting as a Lewis Base.
\[Al(OH)_3 + OH^- \rightarrow Al(OH)_4^- \label{6}\]
- where Al(OH)3 is acting as an Lewis Acid.
Note how the amphoteric properties of the Al(OH)3 depends on what type of environment that molecule has been placed in.
References
- Cycloaddition on Ge(100) of the Lewis Acid AlCl3. Soon Jung Jung,, Young-Sang Youn,, Hangil Lee,,, Ki-Jeong Kim,,, Bong Soo Kim, and, Sehun Kim,. Journal of the American Chemical Society 2008 130 (11), 3288-3289
- Fluorescence Maxima of 10-Methylacridone? Metal Ion Salt Complexes: A Convenient and Quantitative Measure of Lewis Acidity of Metal Ion Salts. Shunichi Fukuzumi and, Kei Ohkubo. Journal of the American Chemical Society 2002 124 (35), 10270-10271.
- Harwood, William S., F. G. Herring, Jeffry D. Madura, and Ralph H. Petrucci. General Chemistry Principles and Modern Applications. 9th ed. New Jersey: Prentice Hall, 2007. 695-96.
Contributors and Attributions
- Adam Abudra (UCD), Tajinder Badial (UCD)

