Polyprotic Acids And Bases
- Page ID
- 1319
The name "polyprotic" literally means many protons. Therefore, in this section we will be observing some specific acids and bases which either lose or accept more than one proton. Then, we will be talking about the equations used in finding the degree of dissociation. Finally, with given examples, we will be able to approach problems dealing with polyprotic acids and bases.
Introduction
Polyprotic acids are specific acids that are capable of losing more than a single proton per molecule in acid-base reactions. (In other words, acids that have more than one ionizable H+ atom per molecule). Protons are lost through several stages (one at each stage), with the first proton being the fastest and most easily lost. Contrast with monoprotic acids in section Monoprotic Versus Polyprotic Acids And Bases.
| Common Polyprotic Acids | Formula | Strong/Weak Acid | Number of Ionizable Hydrogens | Ka1 | Ka2 | Ka3 |
|---|---|---|---|---|---|---|
| Sulfuric acid | H2SO4 | Strong | 2 (diprotic) | Very Large | 1.1E-2 | |
| Sulfurous acid | H2SO3 | Weak | 2 (diprotic) | 1.3E-2 | 6.2E-8 | |
| Phosphoric acid | H3PO4 | Weak | 3 (triprotic) | 7.1E-3 | 6.3E-8 | 4.2E-13 |
| Carbonic acid | H2CO3 | Weak | 2 (diprotic) | 4.4E-7 | 4.7E-11 | |
| Hydrosulfuric acid or Hydrogen sulfide | H2S | Weak | 2 (diprotic) | 1.0E-7 | 1E-19 | |
| Oxalic acid | H2C2O4 | Weak | 2 (diprotic) | 5.4E-2 | 5.3E-5 | |
| Malonic acid | H2C3H2O4 | Medium Strong | 2 (diprotic) | 1.5E-3 | 2.0E-6 |
From the table above, we see that sulfuric acid is the strongest.
Ionization Constant
It is important to know that K1>K2>K3, where K stands for the acidity constant or acid ionization constant (first, second, and third, respectively). These constants are used to measure the degree of dissociation of hydrogens in the acid. For a more in depth discussion on this, go to Ionization Constants.
To find Ka1 of Hydrosulfuric acid (H2S), you must first write the reaction:
\[H_2S \rightleftharpoons H^+ + HS^- \nonumber \]
Dividing the products by the reactants, we then have:
\[K_{a1} = \dfrac{[H^+] [HS^-]}{ [HS-]} \nonumber \]
To find Ka2, we start with the reaction:
\[HS^- \rightleftharpoons H^+ + S_2^- \nonumber \]
Then, like when finding \(K_{a1}\), write the products over the reactants:
\[K_{a2} = \dfrac{[H^+] [S_2^-]}{[HS^-]} \nonumber \]
From these reactions we can observe that it takes two steps to fully remove the H+ ion. This also means that this reaction will produce two equivalence points or stoichiometric points. The equivalence point, by definition, is the point during an acid-base titration in which there has been equal amounts of acid and base reacted. If we were to graph this, we would be able to see exactly just what two equivalence points looks like. Let's check it out:
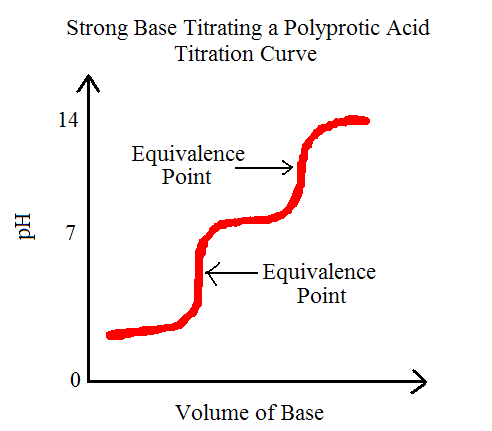
Note the multiple equivalence points and notice that they are almost straight lines at that point, indicating equal added quantities of acid and base.
Titrations
In strong acid + strong base titrations, the pH changes slowly at first, rapidly through the equivalence point of pH=7, and then slows down again. If it is being titrated in a strong acid, the pH will go up as the base is added to it. Conversely, if it is in a strong base, the pH will fall down as acid is added.
- In strong acid + weak base titrations, the pH changes slowly at the equivalence point and the pH equals the pKa of the acid. The pH is below 7.
- For the weak acid + strong base, the pH is above 7 at the equivalence point.
- If there is strong acid or strong base left over after the equivalence point, this can be used to find the pH of the solution.
Next, let's take a look at sulfuric acid. This unique polyprotic acid is the only one to be completely deprotonated after the first step:
\[H_2SO_{4(aq)} + H_2O_{(l)} \rightleftharpoons H_3O^+_{(aq)} + HSO^-_{4(aq)} \nonumber \]
Now let's try something a little harder. The ionization of phosphoric acid (three dissociation reactions this time) can be written like this:
Start with H3PO4:
\[K_{a1}: H_3PO_{4(aq)} \rightleftharpoons H^+_{(aq)} + H_2PO^-_{4(aq)} \nonumber \]
\[K_{a2} : H_2PO^-_{4(aq)} \rightleftharpoons HPO_{4(aq)} + H^+_{(aq)} \nonumber \]
\[K_{a3} : HPO^-_{4(aq)} \rightleftharpoons H^+_{(aq)} + PO^{3-}_{4(aq)} \nonumber \]
So from these above reactions we can see that it takes three steps to fully remove the H+ ion. This also means that this reaction will produce three equivalence points. Polyprotic Bases are bases that can accept at least one H+ ion, or proton, in acid-base reactions.
| Common Polyprotic Bases | Formula | Strong/Weak Base | Diprotic/Triprotic Base |
|---|---|---|---|
| Phosphate ion | PO43- | Weak | Triprotic |
| Sulfate ion | SO42- | Very Weak | Diprotic |
| Carbonate ion | CO32- | Strong | Diprotic |
First, start with the reaction A3- + H2O ? HA2- + OH-
Kb1= [OH-][HA2-]/[A3-]=KW/Ka3
Then, we plug in the products over the reactants:
HA2- + H2O ? H2A- + OH-
Kb2 = [OH-][H2A2-]/[HA2-]=KW/Ka2
Finally, we are left with the third dissociation, or Kb3:
H2A- + H2O ? H3A + OH-
Kb3 = [OH-][H3A]/[H2A-]=KW/Ka1
References
- Petrucci, et al. General Chemistry: Principles & Modern Applications: AIE (Hardcover). Upper Saddle River: Pearson/Prentice Hall, 2007.
Contributors
- Natalie Kania

