Fission Chain Reaction
- Page ID
- 1481
Nuclear Chain Reactions are a simple, yet powerful method which to produce both constructive and destructive forces. Only understood to a significant degree within the last century, nuclear chain reactions have many practical uses in the modern era. Chain reactions can be addressed into two categories: first, controlled (like a nuclear power plant) and uncontrolled (an atomic bomb). Both are motivated by fission reactions, which are elaborated in this section.
- Fission: The event wherein a larger nucleus breaks apart into smaller nuclei, presumably, but not always, more stable.
- Transuranium / Transuranic: Any element with atomic number (z) greater than 92.
- Mega Electron Volt (MeV): The unit of measurement used to express the loss in mass of a given nuclear sample, equal to \(1.6022 \times 10^{-13} \;J\).
- Excitation Energy: Energy required to change the quantum state of a nucleus.
- Fissile: Element that can fission through neutron capture.
- Fertile: Element that can through neutron capture become a fissile material.
- Heavy Water: D2O water made with heavier isotope of hydrogen.
- Chance Fission: Process of energetics which atoms produce fission (marked by 1st, 2nd, 3rd... etc.).
Introduction
Nuclear chain reactions are one of the modern applications of the fission process. Capitalizing upon the large amounts of energy, nuclear chain reactions can be used for constructive and destructive means. To understand how a chain reaction operates, it is best to search in depth of the nature of fission reactions. By understanding fission reactions, a chain reaction is then just an extrapolation of the excess particles produced by fission for a large quantity of an element.
The neutron was discovered by Sir James Chadwick in 1932. In 1939 atomic fission had been discovered by Lise Meitner (1878 to 1968) with her nephew Otto Frisch and was documented in her paper Disintegration of Uranium by Neutrons. A New Type of Nuclear Reaction where the term fission was coined. On December 2, 1942 Dr. Enrico Fermi, a Nobel Prize winner, produced the first sustained chain reaction at the University of Chicago. His assumption was that said reactions produce transuranium products. Otto Hahn and F. Strassmann reproduced Fermi's experiments and found contrary. By 1943 the first controlled chain reaction had taken place and by 1945, the first atomic weapon was produced. By 1951 nuclear chain reactions took a more practical route and became a means to produce electric power.
Chain Reaction
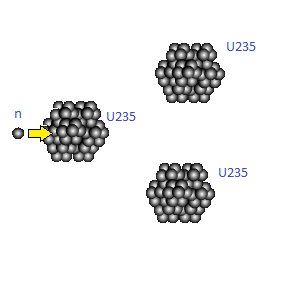
Chain Reactions are basically fission reactions which through the products produce more chain reactions. One of the most well-known and useful examples of a chain reaction is of U235 which is used to harness nuclear energy. For U235 on average 2.5 neutrons are emitted, starting on average two more fission reactions. Below is a simple fission process:
.png?revision=1)
.png?revision=1)
.png?revision=1)
.png?revision=1)
.png?revision=1)
The elements which the daughter nuclei are composed of is based upon probability (compare the fragments of Figures 4 and 5).
Understanding Fission
Understanding Fission is the key for chain reactions. Hence, below is a brief overview of the different aspects of fission. Below are scientific approaches to fission reactions, and the process which fission is produced. Fission Products can be produced by alpha, gamma, beta, charged particles, and through spontaneous reactions. A fission product is affected by the initial mass of nuclide, and excitation energy (expressed in MeV).
Fission Product Yields
Yield is the result is for any fission event, and is highly motivated by probability. This section covers experimental yields and charge yields. The yield can be expressed in two different experimental categories:
Absolute Yield
which can be expressed by the equation:
\(Y{(\%)} = \left(\dfrac{N}{P}\right)*{100}\)
Where Y is the absolute yield, N is the number of atoms a given nucleus formed, and P is the number of fission events. Absolute Yield can be broken down into three different categories:
- Independent
- Cumulative
- Total
- Independent Yield: The probability that a given isotope will form directly.
\(A + n \rightarrow B + C\)
- Cumulative Yield: The probility that a given isotope will form directly (via independent yield) and indirectly by decay of a parent nuclide.
\(A + n \rightarrow B + C\)
\(A \rightarrow D + n\)
\(D \rightarrow E + \beta\)
\(E \rightarrow C + \alpha\)
(this process is through natural decay, where Element A decomposes to Element C)
- Total Yield: Probability that final product of the radioactive decay chain for a specific mass will form.
Note: Sum of total yields for binary fission will be 200%
Relative Yield
Relative Yield \( \gamma_i\) is the ratio of absolute yields formed in fission, which can be expressed through the equation:
\(\gamma_i = \dfrac{Y_i}{Y}\)
where \(\gamma_i\) is the relative yield, Yi is the absolute yield of specific nuclide, which is daughter to a specific nuclei Y also expressed as a yield. Relative yield also has respective independent, cumulative, total yields.
Charge Yield
An approximation of charge ratio (Z/A) is the same for both daughter nuclides as it was for the parent atom. The distribution of charge for daughter nuclides for a fixed mass A can be approximated with the equation:
\(fragment\;charge = e^{-\dfrac{(Z-Z_p)^2}{c}}\)
where Z is proton number, Zp is approximately the charge ratio of the parent nuclide , and c is a number between 0.8 and 1.0 (depending on fission reaction).
Trends of Fission Products
Fission products behave in specific trends, for example: fission is almost always a binary process, as ternary fission is on average 1000 less times probable (In most cases Ternary fission will appear for 1 to 3 events per 1000). Being able to predict the outcome of a fission event is vital for harvesting nuclear energy from power plants. Other trends that can be found is the nature of symmetric and asymmetric fission products. Fission will be symmetric or asymmetric depending on how many nucleons are required to form closed shells. Symmetric peaks can coexist in asymmetric peaks, such as Ra226. Also, a symmetric mass distribution leads to an asymmetric fission process. Note that asymmetric and Symmetric fission are both present in heavy nuclides.
Asymmetric Fission Products: The preferred form of fission leading to two daughter nuclides of unequal masses. Asymmetric fission has an average yield 600 times greater than symmetric fission. Number of outer nucleons drastically affects shape of nucleus, which in turn may change the nature of fission symmetry. The probability of asymmetric fission is high for fission motivated by thermal neutrons (such as U233, U235, Pu239).
Symmetric Fission Products:Preferred for spontaneous reactions (such as Th232, U238, Cm242, and Cf252), symmetric fission is caused by the preference in atomic nuclei to follow the 'magic numbers' most commonly of 50 proton 82 neutron nucleus. Symmetric Produces two daughter nuclides of equal or near equal masses. The range for atomic mass numbers that will be the product of symmetric fission are in the range of 127 to 139 amu. Symmetric fission also is preferred for high excitation energies (at or over 40 MeV). As a function of initial excitation energy, the probability of symmetric fission increases more rapidly than asymmetric fission. Note that multiple combinations are possible for fission chain reactions.
Probability of Fission
An unstable nucleus by the liquid drop model can be expressed in the inequality:
\(\dfrac{Z^2}{A} > {18}\)
Which for any proton number Z and Atomic Mass A, holding this inequality to be true will release energy through symmetric fission. A notable exception is Zr50 which upon the border of this inequality is stable. Although bear in mind that the above inequality is limited because of binding energy and therefore is extremely slow if not impossible. A far more probable inequality would is:
\(\dfrac{Z^2}{A} > {47}\)
Which the second inequality holds for half-lives for super heavy atoms. Note that spontaneous Fission is extremely rare for even radioactive samples.
Favored Reactions
Neutron binding energy is approximately 6 to 7 MeV. Neutron emission of atoms is favored over the fission process when energetically favorable. For the sake of uranium, the neutron emission and fission thresholds are only a few MeV apart. Thus odd-neutron atoms are 'slow neutron fission' and even neutron atoms are 'fast neutron fission'. With this implication it can be deduced that with enough initial excitement energy, multi neutron emission instead of fission is possible.
Fission According to 'Chance'
One of the intriguing portions of operating fission according to chance is the objective of producing more than one neutron before the fission reaction takes place. Hence, is a competition between binding energies of the molecule and neutrons. For example of a second neutron binding energy is greater than the first neutron binding energy, then second chance fission thresholds occur before second neutron emission.
Kinetics of Fission
The major energy of fission lies in the kinetics of the daughter nuclides. The magnitude of the kinetic energy is equal to the coulomb potential energy of fragments. The rest of the energy is released in excitation energy, which is usually neutron emission, and finally gamma radiation for the residual energy. Yield of neutrons that accompany fission is usually a linear relationship expressed as: 1 neutron to 8.5 MeV. A neutron on average carries 5.5 MeV in binding energy and 1.5 MeV in Kinetic energy. Hence, 7 MeV. Neutron yield of lighter fragments is three times greater than heavier fragments.
Note: Excitation energy is placed more into fragments and thus neutron emission than kinetics.
Controlled Chain Reactions
Nuclear Reactors
One of the best examples of Chain reactions is within nuclear power plants. As a fact, nuclear power provides 17 percent of the world's electricity. There are many types of reactors, which all use realatively the same materials, and components to complete a nuclear fission chain reaction.
Terms of Neutron Chain Reaction
- Scattering: When the neutron bounces off the desired fissionable nucleus.
- Leakage: When the neutron does not impact a nucleus, thus not producing a reaction (escapes).
- Capture: When the neutron is absorbed into the nucleus, but said nucleus does not react.
- Thermal Neutron: Neutron with low kinetic energy, less than 0.1 electron volts (eV). More efficient for fission process.
- Fast Neutron: Neutron as a product of fission, with high kinetic energy of 10 million electron volts (eV).
- Critical Mass: The quantity of a radioactive element to sustain a chain reaction.
- Subcritical: Any quantity of a radioactive element below critical mass.
Components of Reactors
- Fuel Element (or Fuel Rod): Contains the fissile material, typically uranium or plutonium, which is used as the fuel to undergo fission and provide the nuclear energy. The fissile material is encased in a solid cladding, made of Zircalloy, to contain both the fuel and the resulting fission products and keep then from escaping into the moderator, coolant, or anywhere outside the cladding. The moderator and coolant flows between the fuel elements (or rods) moderating the neutrons and carrying away the heat. The region inside the nuclear reactor where the fuel elements undergo fission to generate heat is called the nuclear reactor core.
- Moderator: Slows down fast neutrons to thermal energy range. Moderators must be light materials to slow down neutrons without causing capture. Some moderators are: water, carbon, heavy water.
- Coolant: Absorbs and removes the heat produced by nuclear fission. In most current commercial-scale nuclear reactors, purified regular water, called light water, is used as the coolant. Some other coolants are: heavy water, carbon dioxide or helium gas, or molten metals such as sodium, lead, or bismuth.
- Control Rods: Absorbs neutrons, designed to reduce the amount of neutrons available to continue the chain reaction. The control rods, interspersed between the fuel elements in the reactor core, can be inserted into or out of the core as needed to control conditions or shut down the reactor. Some materials used for control rods are: boron, silver, indium, cadmium, or hafnium.
Types of Reactors
- PWR: Pressurized Water Reactor, as of 2003, 212 were active worldwide. Water is both the coolant and moderator and is kept at a high pressure 70 to 150 atm.
- BWR: Boiling Water Reactor, like PWR, water is both the coolant and moderator. Although the water is kept at a lower pressure 70 atm and thus produces steam. The steam directly powers the turbine, thus simplifying the design. The only downside is that over time the turbine accumulates radioactivity (Nitrogen 17 with a half-life of seven seconds).
- Breeder Reactor: Uses both fissile and fertile elements to produce reaction. Because of this, breeder reactors can use materials that are more widely available. Operates by using fast neutrons to convert fertile elements into fissile elements.
Uncontrolled Chain Reactions
The other spectrum of chain reactions, which produce enough energy to cause an explosion. Below are destructive chain reactions by accident.
- Chernobyl: A level 7 on the Nuclear Event Scale, Chernobyl represents a chain reaction gone awry. During an experiment which through numerous errors, the coolant was turned off eventually burning the carbon control rods. A complete meltdown, radioactive smoke spread across Europe causing extensive damage.
- Three Mile Island: In Middleton Pennsylvania 1979, the Three Mile Island reactor experienced a level 5 Nuclear Event. The chain reaction stopped because of too few slow neutrons. Although the chain reaction ceased, the fission process continued sending the fuel rods to extreme temperatures. A small fissure developed in one of the reactors, thus sending radioactive steam into the atmosphere.
- Fukushima BWR reactors: Due to a severe earthquake and tsunami in Japan in March 2011, several BWR nuclear reactors at the Fukushima power plant lost electrical power for cooling, underwent explosions, and suffered reactor core damage as workers eventually pumped seawater into the reactors to cool them down and limit any further damage.
International Nuclear Event Scale (INES): The scale is written by degree of danger (lower in the list is greater danger)
| 0 | No safety significance | Deviation |
| 1 | Anomaly | " " |
| 2 | Incident | Incident |
| 3 | Serious incident | " " |
| 4 | Accident without significant off-site risk | " " |
| 5 | Accident with off-site risk | Accident |
| 6 | Serious Accident | " " |
| 7 | Major Accident | " " |
References
- Nordborg, Claes, Hans Riotte, Miroslav Hrehor, Ted Lazo, Stefan Mundigl, Peter Wilmer, Julia Schwartz, Carol Kessler, Jaques De La Ferte, and Robert Price. "Overview of Nuclear Energy Today, Basic Principles of Nuclear Energy." Nuclear Energy Today. Ed. Cynthia Picot. Paris, France: Nuclear Energy Agency, Organisation for Economic Co-operation and Development, 2003.
- Print. Petrucci, Ralph H., Jeffrey D. Madura, Carey Bissonnette, and F. Geoffrey Herring. "25-8 Nuclear Fission." General Chemistry: Principles and Modern Applications. 10th ed. Upper Saddle River: Pearson Canada, 2011. 1137-140. Print.
- Wilets, Lawrence. Theories of Nuclear Fission. Oxford: Clarendon P, 1964. Print.
- Zysin, Ju A., A. A. Lbov, and L. I. Sel'chenkov. Fission Product Yields and Their Mass Distribution: Transl. from Russian. New York, 1964. Print.
Problems
Easy Problems
- (True/False) Fission cannot be induced by gamma radiation because it is too small to affect nucleus.
- After five successful binary fission events, how many daughter fragments are produced?
Moderate Problems
3. A sample of Element Lol produced 15 fragment nuclides in 45 fission events, what is the absolute Yield Y(%)?
4. A new isotope of Xenon has been discovered with an atomic mass of 62 by the practical equation for spontaneous fission, will Xe62 undergo spontaneous fission? (Ignoring that this is absolutely impossible)
Hard Problems
5. For a yield of a given mass, would the end link in a radioactive chain equal the total yield?
Answers
- False.
- \(2^5 = 32\)
- \(\dfrac{15}{45}*{100} = {.333}*{100} = {33.3}\%\)
- \(\dfrac{54^2}{62} = 47.03 > 47\)
- By using the definition of Cumulative Yield and Total Yield the answer would be YES.

