Cohesive and Adhesive Forces
- Page ID
- 1499
Cohesive and adhesive forces are associated with bulk (or macroscopic) properties, and hence the terms are not applicable to the discussion of atomic and molecular properties. When a liquid comes into contact with a surface (such as the walls of a graduated cylinder or a tabletop), both cohesive and adhesive forces will act on it. These forces govern the shape which the liquid takes on. Due to the effects of adhesive forces, liquid on a surface can spread out to form a thin, relatively uniform film over the surface, a process known as wetting. Alternatively, in the presence of strong cohesive forces, the liquid can divide into a number of small, roughly spherical beads that stand on the surface, maintaining minimal contact with the surface.
Adhesive and Cohesive Forces
The term "cohesive forces" is a generic term for the collective intermolecular forces (e.g., hydrogen bonding and van der Waals forces) responsible for the bulk property of liquids resisting separation. Specifically, these attractive forces exist between molecules of the same substance. For instance, rain falls in droplets, rather than a fine mist, because water has strong cohesion, which pulls its molecules tightly together, forming droplets. This force tends to unite molecules of a liquid, gathering them into relatively large clusters due to the molecules' dislike for its surroundings.
Similarly, the term "adhesive forces" refers to the attractive forces between unlike substance, such as mechanical forces (sticking together) and electrostatic forces (attraction due to opposing charges). In the case of a liquid wetting agent, adhesion causes the liquid to cling to the surface on which it rests. When water is poured on clean glass, it tends to spread, forming a thin, uniform film over the glasses surface. This is because the adhesive forces between water and glass are strong enough to pull the water molecules out of their spherical formation and hold them against the surface of the glass, thus avoiding the repulsion between like molecules.
Macroscopic Effects of Cohesive and Adhesive Forces
When a liquid is placed on a smooth surface, the relative strengths of the cohesive and adhesive forces acting on that liquid determine the shape it will take (and whether or not it will wet the surface). If the adhesive forces between a liquid and a surface are stronger, they will pull the liquid down, causing it to wet the surface. However, if the cohesive forces among the liquid itself are stronger, they will resist such adhesion and cause the liquid to retain a spherical shape and bead the surface.
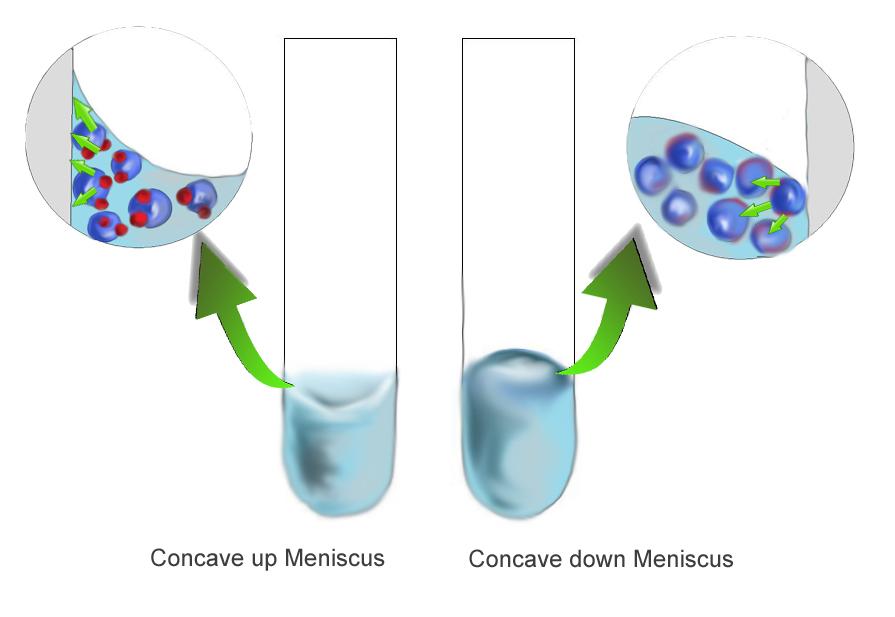
Case I: The Meniscus
The meniscus is the curvature of a liquid's surface within a container, such as a graduated cylinder. However, before we explain why some liquid have a concave up meniscus while others share a concave down meniscus, we have to understand the adhesive forces at work of surface tension. Water, for example, is a polar molecule that consists of a partial positive charge on the hydrogens and a partial negative charge on the oxygen. Thus, within liquid water, each molecule's partial positive charge is attracted to its neighbor's partial negative charge. This is the origin of the cohesive forces within the water. Water molecules buried inside the liquid is then being pulled and pushed evenly in every direction, producing no net pull. Meanwhile, the molecules on the surface of the liquid, lacking pulling forces in the upward direction, thus encompass a net downward pull.
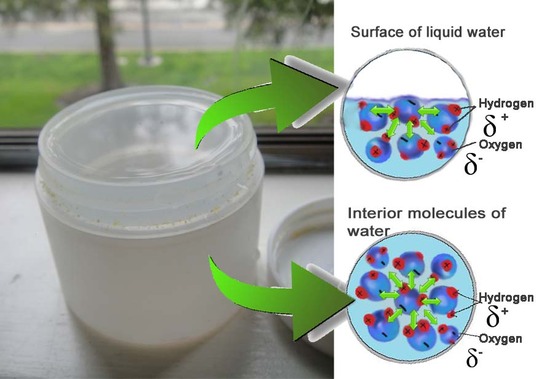
How does this cohesive force create both a concave up and concave down surface then? The answer is in its relationship to the adhesive forces between the water molecules and the container's surface. When the cohesive force of the liquid is stronger than the adhesive force of the liquid to the wall, the liquid concaves down in order to reduce contact with the surface of the wall. When the adhesive force of the liquid to the wall is stronger than the cohesive force of the liquid, the liquid is more attracted to the wall than its neighbors, causing the upward concavity.
Case II: Tears of Wine
In agitated glasses of wine, droplets of wine seemingly "float" above the meniscus of the liquid and form "tears." This age-old phenomenon is the result of surface tension and cohesive and adhesive forces. Alcohol is more volatile than water. As a result, "evaporation of alcohol produces a surface tension gradient driving a thin film up along the side of a wine glass" (Adamson). This process is called the "solutal Marangoni effect."2 Due to adhesive forces, some waters clings to the walls of the glass. The "tears" form from the cohesive forces within the water holding it together. It is important to note that the surface tension gradient is "the driving force for the motion of the liquid" (Gugliotti), but the actual formation of the tears is a result of cohesive and adhesive forces.

Problems
- Name two examples where the cohesive force dominates over the adhesive force and vice versa.
- When in a glass graduated cylinder, water presents an upwardly concave meniscus. However, when water is filled to the tip of the cylinder, the water level could maintain higher than the wall of the cylinder without pouring out resembling a concave down meniscus. Use the principles of cohesive and adhesive forces to explain this situation.
- Explain why a water strider can glide on the water with the knowledge of cohesion in water.
- Propose different types of forces that adhesive forces can build on.
Answers
- When cohesive force is stronger than the adhesive force: concave up meniscus, water forms droplets on the surface. When adhesive force is stronger than the cohesive force: concave down meniscus, the surfaces are covered by the wetting agent, the last drops of liquid in the bottle always refuse to come out.
- Since water forms a concave up meniscus, the adhesion of the molecules to the glass is stronger than the cohesion among the molecules. However, in the absence of the adhesive force (when water reaches the tip of the glass), the cohesive force remains present. Thus cohesive force alone proves that it can still hold itself in place without pouring out of the cylinder. This example emphasizes the importance of that cohesive force and adhesive forces do not simply cancel each other out, yet it is the difference between the two that determines the characteristic of the liquid.
- This problem addresses once again the concept of surface tension. Because the cohesion of the water is built on the weak intermolecular forces of the water, when a water strider stepson to the surface, an extra energy will be necessary to overcome to break those bonds to increase surface area. Moreover, since the gravitational pull down on the water strider cannot overcome the activation energy to break these intermolecular forces, the water strider is able to glide freely on the water.
- Complementary shape, chemical bonds form, weak intermolecular forces such as H-bonding or Van der Waals forces.
References
- Petrucci, et al. General Chemistry: Principles & Modern Applications: AIE (Hardcover). Upper Saddle River: Pearson/Prentice Hall, 2007.
- Gugliotti, Marcos. "Tears of Wine." Journal of Chemical Education 81.1 (2004): 67-68. Web. 9 Mar. 2010.
- Adamson, A. W.; Gast, A. P. Physical Chemistry of Surfaces, 6th ed.; John Wiley and Sons: New York, 1997; p 371.
Contributors and Attributions
- Cameron Tracy, Ling Xie, Irene Lym, Henry Li

