Surface Tension
- Page ID
- 1502
Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water vs. gasoline) or solutes in the liquid (e.g. surfactants like detergent), each solution exhibits differing surface tension properties. Whether you know it or not, you already have seen surface tension at work. Whenever you fill a glass of water too far, you may notice afterward that the level of the water in the glass is actually higher than the height of the glass. You may have also noticed that the water that you spilled has formed into pools that rise up off the counter. Both of these phenomena are due to surface tension.

Molecular Perspective
In a sample of water, there are two types of molecules. Those that are on the outside, exterior, and those that are on the inside, interior. The interior molecules are attracted to all the molecules around them, while the exterior molecules are attracted to only the other surface molecules and to those below the surface. This makes it so that the energy state of the molecules on the interior is much lower than that of the molecules on the exterior. Because of this, the molecules try to maintain a minimum surface area, thus allowing more molecules to have a lower energy state. This is what creates what is referred to as surface tension. An illustration of this can be seen in Figure \(\PageIndex{1}\).
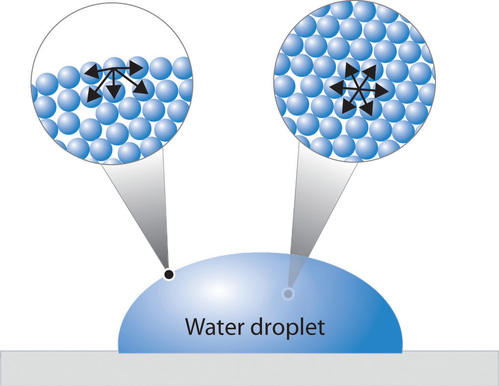
The water molecules attract one another due to the water's polar property. The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to "stick" together. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Same goes for other liquids, even hydrophobic liquids such as oil. There are forces between the liquid such as Van der Waals forces that are responsible for the intermolecular forces found within the liquid. It will then take a certain amount of energy to break these forces, and the surface tension. Water is one liquid known to have a very high surface tension value and is difficult to overcome.
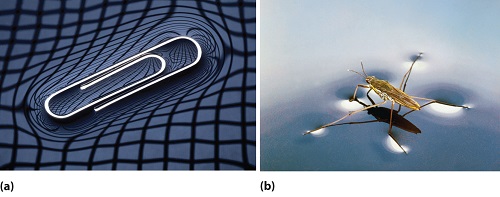
Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (Figure \(\PageIndex{2}\)). An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is distributed to its legs. Surface tension also allows for the formation of droplets that we see in nature.
Cohesive and Adhesive Forces
There are several other important concepts that are related to surface tension. The first of these is the idea of cohesive and adhesive Forces. Cohesive forces are those that hold the body of a liquid together with minimum surface area and adhesive forces are those that try to make a body of a liquid spread out. So if the cohesive forces are stronger than the adhesive forces, the body of water will maintain its shape, but if the opposite is true than the liquid will be spread out, maximizing its surface area. Any substance that you can add to a liquid that allows a liquid to increase its surface area is called a wetting agent.
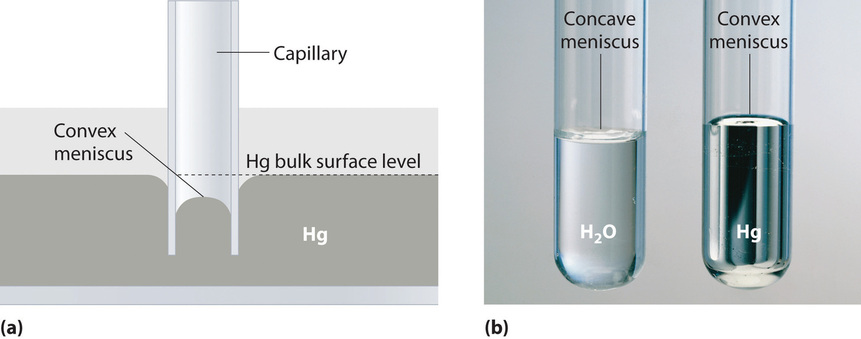
In the lab there are also several important points to remember about surface tension. The first you've probably noticed before. This is the idea of a meniscus (Figure \(\PageIndex{3a}\)). This is the concave (curved in) or convex (curved out) look that water or other liquids have when they are in test tubes. This is caused by the attraction between the glass and the liquid. With water, this causes it to climb up the sides of a test tube. This attraction is amplified as the diameter of the tubes increases; this is called capillary action. This can be seen if you take a tube with a very small diameter (a capillary tube) and lower it into a body of water. The liquid will climb up into the tube, even though there is no outside force. You may have also seen this when you put a straw into a drink and notice that the liquid level inside the straw is higher than it is in your drink. All of this however, requires that the adhesive forces (between the liquid and the capillary surface) be higher than the cohesive forces (between the liquid and itself), otherwise there will be no capillary action or the opposite can even happen. Mercury has higher cohesive forces than adhesive forces, so the level of the liquid will actually be lower in the capillary tubes than compared to the rest of the mercury (Figure \(\PageIndex{3b}\)).
References
- Petrucci, Ralph H., et al. General Chemistry: Principles and Modern Applications. Upper Saddle River, NJ: Prentice Hall, 2007.
Contributors and Attributions
- Benjamin Aldridge, Nivaz Brar

