Colloids
- Page ID
- 1595
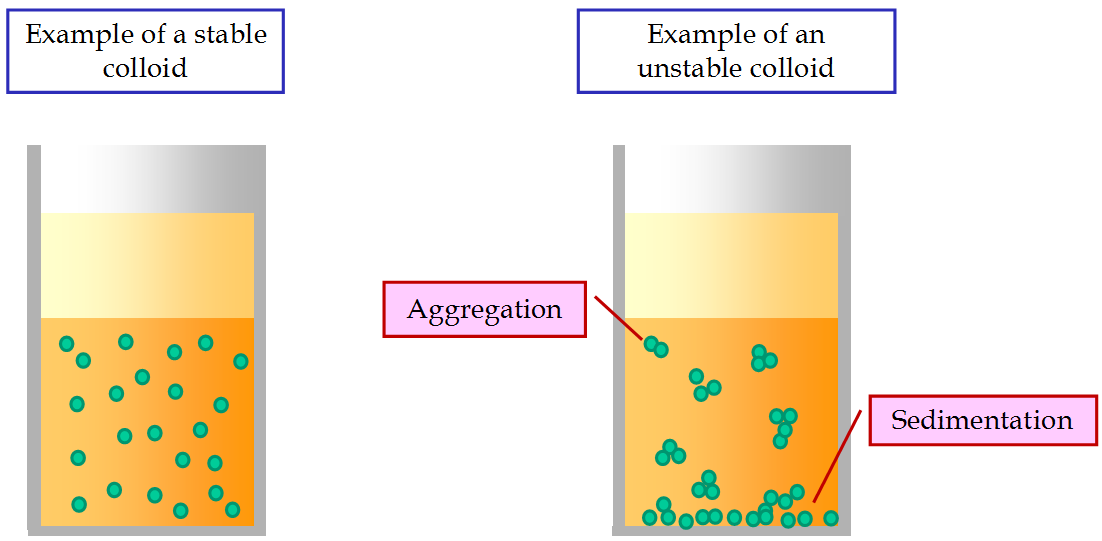
A colloid is one of the three primary types of mixtures, with the other two being a solution and suspension. A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout the solution. These are also known as colloidal dispersions because the substances remain dispersed and do not settle to the bottom of the container. In colloids, one substance is evenly dispersed in another. The substance being dispersed is referred to as being in the dispersed phase, while the substance in which it is dispersed is in the continuous phase.
To be classified as a colloid, the substance in the dispersed phase must be larger than the size of a molecule but smaller than what can be seen with the naked eye. This can be more precisely quantified as one or more of the substance's dimensions must be between 1 and 1000 nanometers. If the dimensions are smaller than this the substance is considered a solution and if they are larger than the substance is a suspension.
Classifying Colloids
A common method of classifying colloids is based on the phase of the dispersed substance and what phase it is dispersed in. The types of colloids includes sol, emulsion, foam, and aerosol.
- Sol is a colloidal suspension with solid particles in a liquid.
- Emulsion is between two liquids.
- Foam is formed when many gas particles are trapped in a liquid or solid.
- Aerosol contains small particles of liquid or solid dispersed in a gas.
When the dispersion medium is water, the collodial system is often referred to as a hydrocolloid. The particles in the dispersed phase can take place in different phases depending on how much water is available. For example, Jello powder mixed in with water creates a hydrocolloid. A common use of hydrocolloids is in the creation of medical dressings.
| Dispersion Medium | Dispersed Phase | Type of Colloid | Example |
|---|---|---|---|
| Solid | Solid | Solid sol | Ruby glass |
| Solid | Liquid | Solid emulsion/gel | Pearl, cheese |
| Solid | Gas | Solid foam | Lava, pumice |
| Liquid | Solid | Sol | Paints, cell fluids |
| Liquid | Liquid | Emulsion | Milk, oil in water |
| Liquid | Gas | Foam | Soap suds, whipped cream |
| Gas | Solid | Aerosol | Smoke |
| Gas | Liquid | Aerosol | Fog, mist |
An easy way of determining whether a mixture is colloidal or not is through use of the Tyndall Effect. When light is shined through a true solution, the light passes cleanly through the solution, however when light is passed through a colloidal solution, the substance in the dispersed phases scatters the light in all directions, making it readily seen. An example of this is shining a flashlight into fog. The beam of light can be easily seen because the fog is a colloid.
Another method of determining whether a mixture is a colloid is by passing it through a semipermeable membrane. The larger dispersed particles in a colloid would be unable to pass through the membrane, while the surrounding liquid molecules can. Dialysis takes advantage of the fact that colloids cannot diffuse through semipermeable membranes to filter them out of a medium.
Problems
- Is dust a colloid? If so, what type is it?
- Is whipped cream a colloid? if so, what type is it?
- What does Sol mean?
- When hit by light what happens to a colloidal mixture?
- What is the mixture considered if the particles are larger than the particles of a colloidal substance
Answers
- Dust is a colloid if suspended in air. It consists of a solid in a gas, so it is a aerosol.
- Whipped cream is a colloid. It consists of a gas in a liquid, so it is a foam.
- Sol is a colloidal suspension with solid particles in a liquid.
- The light is reflected off the large particles and spread out.
- It's considered a suspension if the particles are larger than 1000 nanometers.
References
- Petrucci, et al. General Chemistry: Principles & Modern Applications. 9th ed. Upper Saddle River, New Jersey 2007
Contributors and Attributions
- Jimmy Law (UCD), Abheetinder Brar (UCD)
- Thumbnail: pixabay.com/photos/milk-spra...-food-4755234/

