Chemical Compounds
- Page ID
- 1642
Chemical compounds can generally be classified into two broad groups: molecular compounds and ionic compounds. Molecular compounds involve atoms joined by covalent bonds and can be represented by a variety of formulas. Ionic compounds are composed of ions joined by ionic bonding, and their formulas are generally written using oxidation states.
Molecular compounds are composed of atoms that are held together by covalent bonds. These bonds are formed when electrons are shared between two atoms. The concept of chemical formulas was created to describe many characteristics of molecular compounds in a simple manner. A normal chemical formula encompasses factors such as which elements are in the molecule and how many atoms of each element there are. The number of atoms of each element is denoted by a subscript, a small number that is written to the left of the element.
\[ CH_3COOH \nonumber \]
In the preceding formula, the subscript “3” denotes the fact that there are three hydrogen atoms present in the molecule.
Other types of formulas are used to display more detailed characteristics of molecules.
An empirical formula represents the proportions of atoms in a molecule. It gives important information about a molecule, because it displays the ratios of atoms that are present within the molecule. However, its limitations exist in the sense that it does not represent the exact number of atoms present in the molecule as the molecular formula does. In certain situations, the molecular and the empirical formula can be the same, but in other situations, the molecular formula is a multiple of the ratios of atoms indicated in the empirical formula. Since empirical formulas can be derived from molecular formulas, molecular formulas are generally more useful than empirical formulas.
Empirical vs. molecular compounds
C5H7O is a possible empirical formula, because a ratio of 5:7:1 cannot be simplified any further. In this particular case, the empirical formula could also be the molecular formula, if there are exactly 5 carbon atoms, 7 hydrogen atoms, and 1 oxygen atom per molecule. However, another possible molecular formula for this same molecule is C10H14O2, because while there are 10 carbon atoms, 14 hydrogen atoms, and 2 oxygen atoms present, the ratio 10:14:2 can be simplified to 5:7:1, giving way to the same empirical formula. Additionally, C10H14O2 is not the only possibility of a molecular formula for this molecule; any formula with the same relative proportions of these atoms that can be simplified to a 5:7:1 ratios is a possible molecular formula for this molecule. When given adequate information, the empirical formula and molecular formula can be quantitatively ascertained.
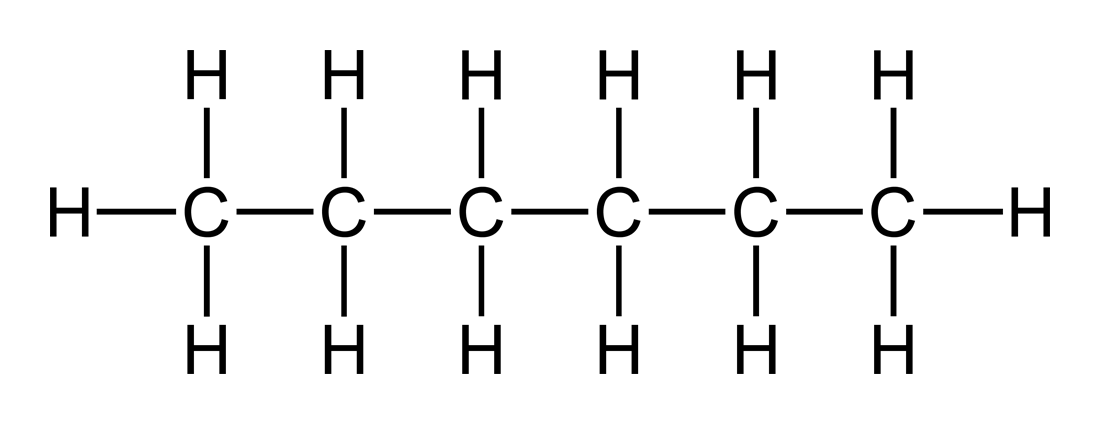
A structural formula is written to denote the details of individual atoms’ bonding. More specifically, it clarifies what types of bonds exist, between which atoms these bonds exist, and the order of the atoms’ bonding within the molecule. Covalent bonds are denoted by lines. A single line represents a single bond, two lines represent a double bond, three lines represent a triple bond, and onwards. A single covalent bond occurs when two electrons are shared between atoms, a double occurs when four electrons are shared between two atoms, etc. In this sense, the higher the number of bonds, the stronger the bond between the two atoms.
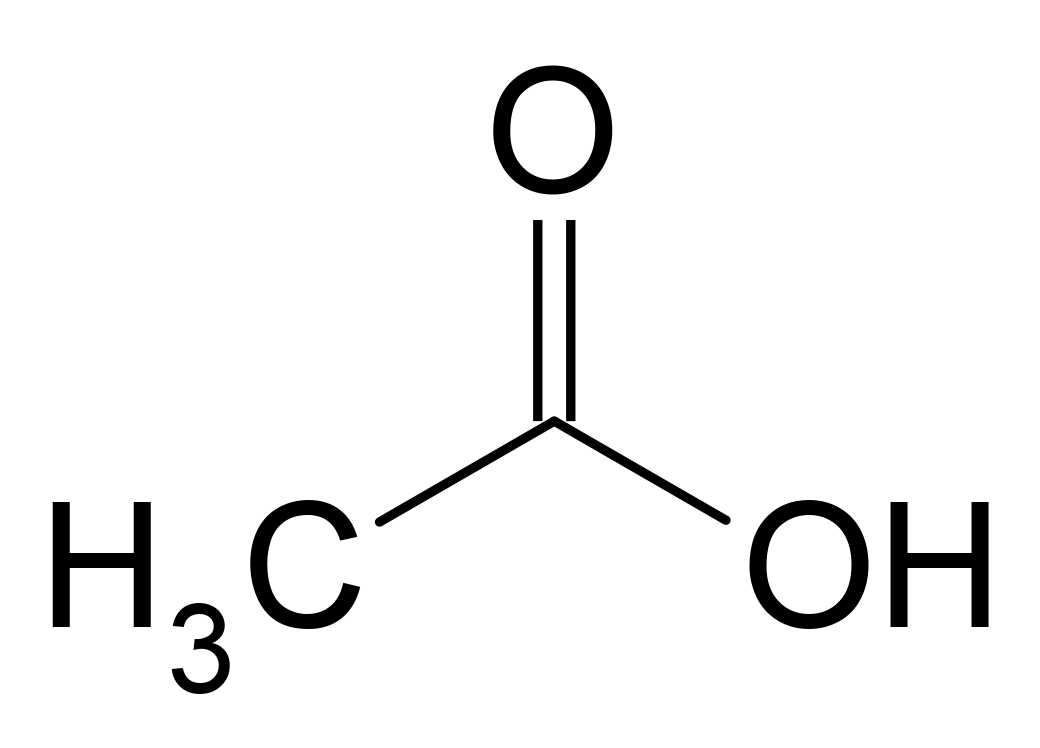
A condensed structural formula is a less graphical way of representing the same characteristics displayed by a structural formula. In this type of formula, the molecule is written as a molecular formula with the exception that it indicates where the bonding occurs.
All the representations discussed thus far have not addressed how to show a molecule’s three-dimensional structure. The two ways to illustrate a spatial structure are through the use of the ball-and-stick model as well as the space-filling model.
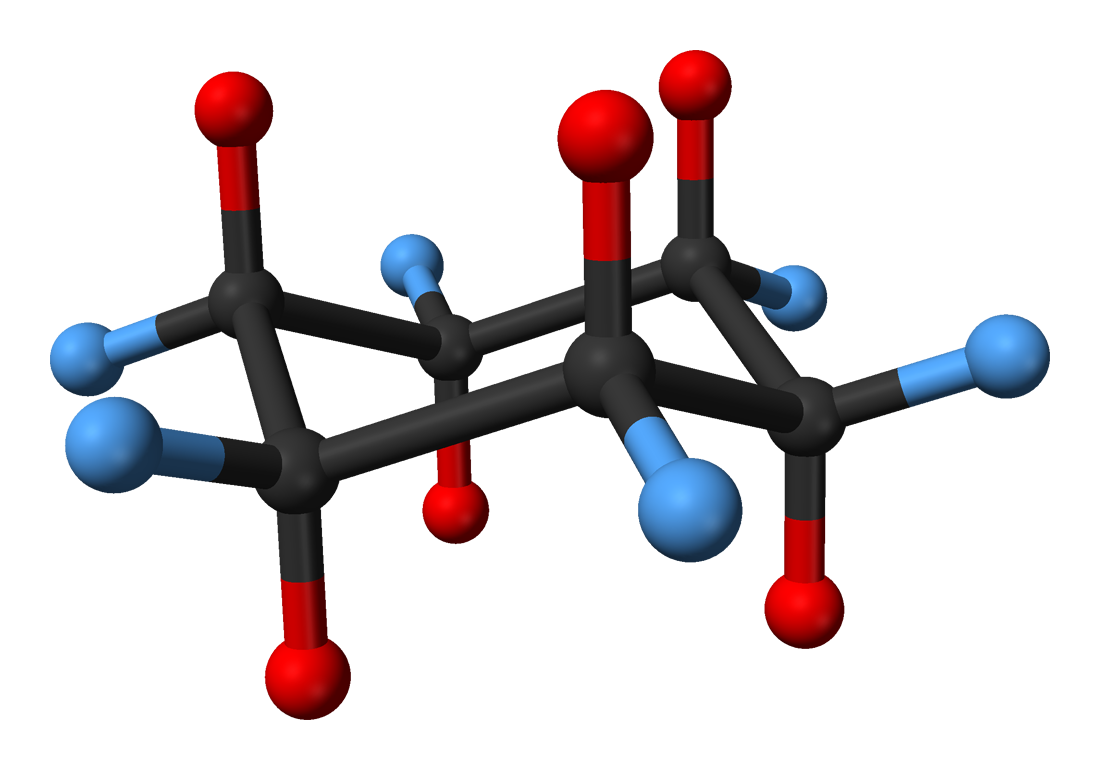
The ball-and-stick model uses balls to spatially represent a molecule. The balls are the atoms in a molecule and sticks are the bonds between specific atoms.

The space-filling model is also a method of spatially displaying a molecule and its characteristics. A space-filling model shows atoms’ sizes relative sizes to one another.
Ionic Compounds
Ionic compounds are composed of positive and negative ions that are joined by ionic bonds. Ionic bonds are generally formed when electrons are transferred from one atom to another, causing individual atoms to become charged particles, or ions. Ions can be referred to as either monatomic or polyatomic. Monatomic ions such as Cl- are composed of only one ion, while polyatomic ions such as NO3- are defined as polyatomic ions. A combination of these ions that forms a compound whose charge is equal to zero is known as a formula unit of an ionic compound. Ionic compounds generally tend to form crystallized salts. They generally have high boiling/melting points, and are good conductors of electricity. The formulas of ionic compounds are always written with the cation first, followed by the anion. The formula can then be completed with reference to the oxidation states of the elements present.
References
- Petrucci, Ralph. Harwood, William. Herring, Geoffrey. Madura, Jeffery. GENERAL CHEMISTRY Principles and Modern Applications 9th Edition. Macmillan Publishing co, New Jersey. 1989.
- Suchocki, J. Conceptual Chemistry: Understanding Our World of Atoms and Molecules. 2nd ed. United Kingdom: Benjamin/Cummings, 2003.

