Internal Energy
- Page ID
- 1955
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
The internal energy of a system is identified with the random, disordered motion of molecules; the total (internal) energy in a system includes potential and kinetic energy. This is contrast to external energy which is a function of the sample with respect to the outside environment (e.g. kinetic energy if the sample is moving or potential energy if the sample is at a height from the ground etc). The symbol for Internal Energy Change is\( ΔU\).
Energy on a smaller scale
- Internal energy includes energy on a microscopic scale
- It is the sum of all the microscopic energies such as:
- translational kinetic energy
- vibrational and rotational kinetic energy
- potential energy from intermolecular forces
| Example |
|---|
|
One gram of water at zero °Celsius compared with one gram of copper at zero °Celsius do NOT have the same internal energy because even though their kinetic energies are equal, water has a much higher potential energy causing its internal energy to be much greater than the copper's internal energy. |
Internal Energy Change Equations
The first law of thermodynamics
ΔU = q+w
where q is heat and w is work
An isolated system cannot exchange heat or work with its surroundings making the change in internal energy equal to zero.
ΔUisolated system = 0
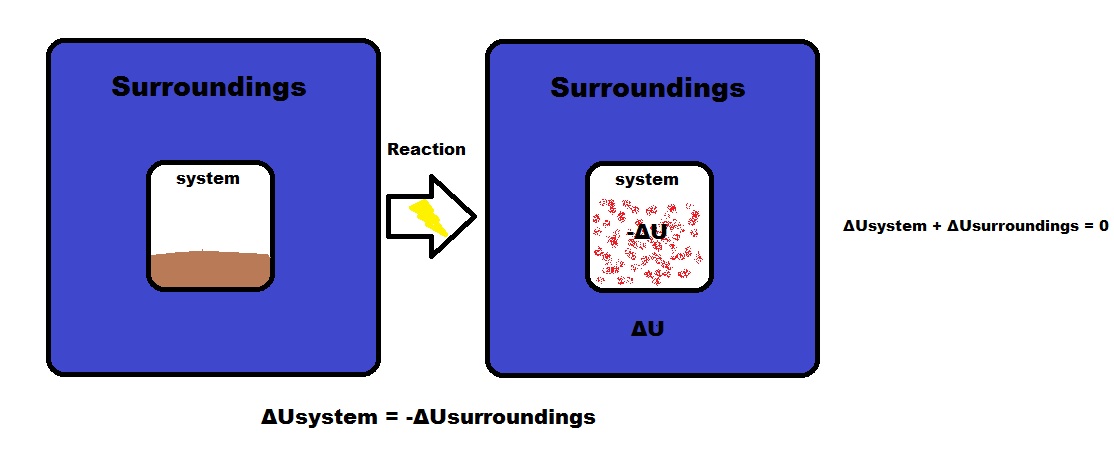
Energy is Conserved
ΔUsystem = -ΔUsurroundings
The signs of internal energy
- Energy entering the system is POSITIVE (+), meaning heat is absorbed, q>0. Work is thus done on the system, w>0
- Energy leaving the system is NEGATIVE (-), meaning heat is given off by the system, q<0 and work is done by the system, w<0
- Since ΔUisolated system = 0, ΔUsystem = -ΔUsurroundings and energy is conserved.
Quick Notes
- A system contains ONLY internal Energy
- a system does NOT contain energy in the form of heat or work
- Heat and work only exist during a change in the system
- Internal energy is a state function
Outside Links
- Levine, Ira N. "Thermodynamic internal energy of an ideal gas of rigid rotors." J. Chem. Educ. 1985: 62, 53.
Contributors and Attributions
- Lorraine Alborzfar (UCD)