2.3 The Direction of Chemical Change
- Page ID
- 32249
What determines whether a reaction will actually occur spontaneously or not? In chemistry, a "spontaneous" reaction is a reaction that will occur on its own - it may be fast or it may be slow.
Na(s) + ½Cl2(g) → NaCl(s) + 411.2 kJ
H2(g) + ½O2(g) → H2O(g) + 242 kJ
| Something that all of these reactions have in common is that they are all exothermic. Energy, like boulders, tends to run downhill. The lower energy state of the products is more stable than the high energy state of the reactants. And everything always likes to go towards the most stable, or lowest energy, state.
| 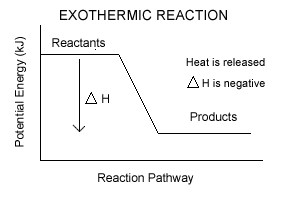 |
| But of course some endothermic reactions that are also spontaneous: Br2(l) + Cl2(g) + 29.3 kJ → 2 BrCl(g) H2(s) + ½O2+ 6.01 kJ → H2O(l) | Take a moment to examine the amount of energy required for these endothermic examples. Not much energy is required. Generally, if a large amount of energy must be supplied (the reaction is highly endothermic), the reaction will likely notbe spontaneous. |
We therefore see that whether or not a reaction is spontaneous depends on more than just the enthalpy change. But, the enthalpy change is, without a doubt, one of the most important factors determining whether or not a reaction will actually be spontaneous. We will discuss the other key factor in Lesson 3.
Complete the assignment before continuing.

